Professor Associado, Universidade Fernando
Pessoa
| Other interactive models: |
| Metabolic pathways: |
|
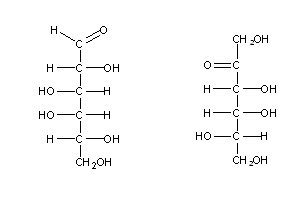
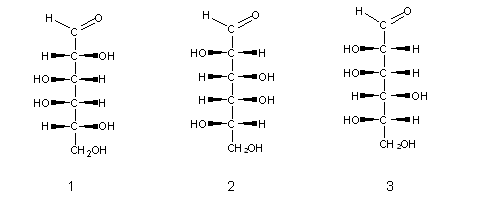
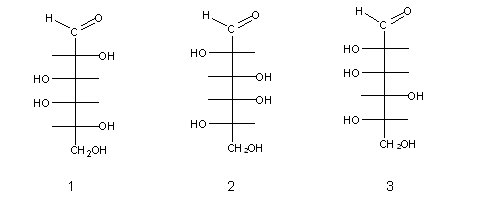
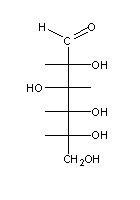
Naturally-occurring monossaccharides have one common feature: when written as Fischer formulae, the las assymetric carbon always has an OH group at its right side . These sugars (e.g. sugar (1) above) are called D-sugars (from Latin dexter, meaning "right"), and their enantiomers are L-sugars (from Latin levo, meaning "left"), e.g. sugars (2) and (3) above.
A monossaccharide may be converted into cyclic forms, through nucleophilic attack of its carbonyl group by one of its hydrocil groups. This reaction is a relevant example of hemiketal occurrence in Biology. These cyclic forms are only stable if the ring has five or more sides (otherwise the bond angles will be too different from the ideal value of ≈ 109o ). In practice, common sugars form either five-sided (furanoses) or six-sided rings (pyranoses). In aqueous solution, cyclic forms are often much more stable than the corresponding linear forms.
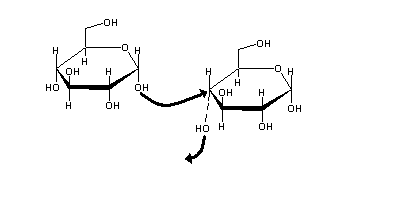
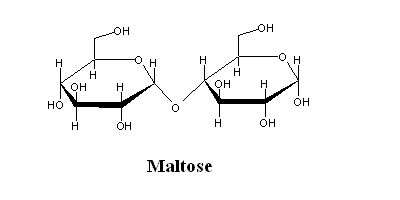
| (Glucose α-1,4 glucose) the product of starch degradation by amylases. |
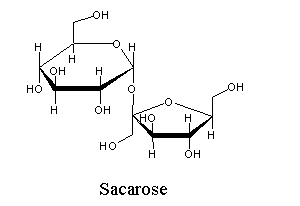
| (Glucose α-1 β-2 fructose) the sugar extracted from sugar-cane. |
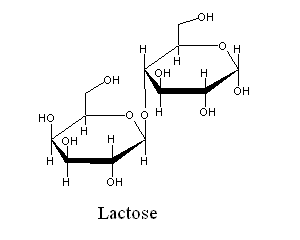
| (Galactose β-1,4 glucose) the sugar present in milk. In most mammals, the enzyme responsible for lactose hydrolysis (lactase) is only synthesized during nursing. Absent lactase, lactose cannot be digested, and it becomes an abundant food source for gut bacteria, thereby causing nausea, vomitting, and diarrhea. Since cattle domestication, several human populations have developped the ability to express lactase throughout adult life. |
 |
Biochemistry,
by Donald Voet & Judith Voet An excellent text. It presents Biochemistry with frequent references to organic chemistry and biochemical logic. Highly reccommended for students of Biochemistry, Chemistry and Pharmaceutical Sciences. |
 |
Biochemistry,
Stryer A widely used classical text, frequently updated and re-issued. |
 |
Textbook
of Biochemistry with Clinical Correlations, Thomas Devlin Strongly advised to students in Nursing, Medicine, Dentistry, etc. Plenty of examples of application of biochemical knowledge to clinical cases. |
 |
Principles
of Biochemistry, Lehninger A widely used classical text, frequently updated and re-issued. |