Effects of activation on the electron transfer between Pyrococcus furiosus hydrogenase and its redox partners
P.J. Silva+*, M.-J.
Amorim+*, P.-L. Hagedoorn*, H. Wassink*,
H. Haaker* and W.R. Hagen*
*Wageningen University,
Department of Biochemistry, Dreijenlaan 3, NL-6703 HA Wageningen, The Netherlands
+CEQUP, Departamento
de Química, Faculdade de Ciências, Rua do Campo Alegre,687,
4169-007 Porto, Portugal
The (sulf)hydrogenase complex from the hyperthermophilic
archaeon Pyrococcus furiosus is a very thermostable abgd heterotetramer
with both hydrogenase activity (borne by the ad subunits) and sulfur reductase
activity (carried by the bg subunits). Upon heat-induced reduction by an
yet unidentified internal substrate it passes through a number of states
some of which are similar to states previously defined for mesophilic hydrogenases.
The complexity of these transitions reflects a combination of temperature-dependent
activation and temperature-dependent reduction potentials which may explain
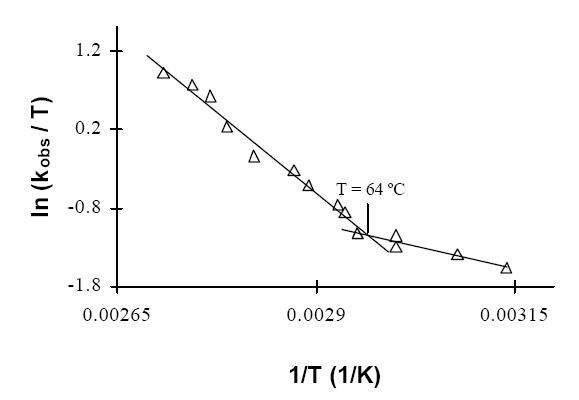
its inactivity at room temperature. The increase of its H2-uptake
activity with temperature reveals a conformational change at 64 ºC
which changes the activation DH from 19.3 kJmol-1
to 68 kJmol-1 and increases the activation DS
from –150 JK-1mol-1 to –4 JK-1mol-1.
Originally, the [4Fe-4S] ferredoxin was proposed
to be the natural redox partner of (sulf)hydrogenase in P. furiosus.
Later this idea was rejected in favor of a model in which ferredoxin gives
electrons to a ferredoxin:NADP+ oxidoreductase (FNOR). The produced
NADPH is then thought to reduce hydrogenase. The FNOR dimer contains three
Fe/S clusters and two FAD. EPR-monitored electrochemical titrations has
allowed the determination of the redox potentials of the Fe/S clusters.
Two clusters show anomalous relaxation behaviour which may be related to
their putative ligands (deduced from analysis of the gene sequence). Each
of the two subunits of this enzyme is homologous to members of a different
class of flavo-iron-sulfur proteins.
Incubation of (sulf)hydrogenase under H2
at 80 ºC revealed a time-dependent activation pattern for H2-uptake
activity. The influence of this process on the electron-transfer ability
between ferredoxin (or FNOR-produced NADPH) and sulfhydrogenase (and its
relevance to the physiological electron-disposal pathway) will be discussed.
Acknowledgments. PJS thanks Fundação para a Ciência e Tecnologia/Praxis XXI for a fellowship. MJA thanks the SOCRATES program for a grant.
Journal of Inorganic Biochemistry (1999), 74, 297