Associate Professor, Universidade Fernando Pessoa
| Other metabolic pathways: |
Enzymes relay the electrons released by substrate oxidation to special molecules we call electron acceptors. Electron acceptors may be organic or inorganic, and the most common examples thereof are NAD+ and FAD. Each of these molecules ca accept two electrons, yielding NADH+H+ and FADH2, respectively. Since cellular amounts of NAD+ and FAD are very small, special mechanisms are needed in order to convert NADH+H+ and FADH2 back into NAD+ and FAD. This is performed through electron transfer from NADH+H+ and FADH2 to other molecules, which may occur through either fermentation or respiration. Contrary to general belief, the distinction between these two processes does not lie on a requirement for O2!
Fermentation
In fermentation, NADH (or FADH2) donates its electrons to a molecule produced by the same metabolic pathway that produced the electrons carried by NADH (or FADH2). For instance, during intense physical exercise by muscles, NADH generated through glycolysis transfers its electrons to pyruvate (an organic molecule produced by glycolysis), yielding lactate.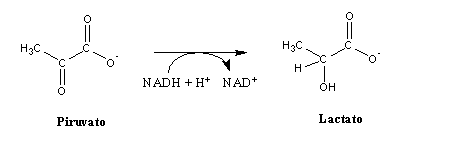
(The relationship between the pH drop in muscles during lactate production and the occurrence of cramps is discussed in detail in these two papers). This process is called lactic fermentation . Many other kinds of fermentation have been found in microorganisms, and the most well-known among these is alcoholic fermentation:
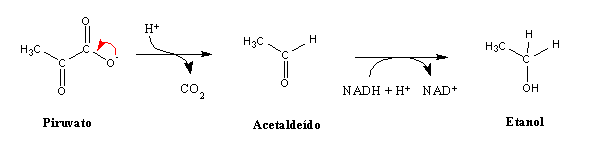
Respiration
In respiration, the final acceptor of NADH (or FADH2) electrons is not a product of the metabolic pathway that released the electrons carried by NADH (or FADH2). Many microorganisms use SO42-, SeO42- ,NO3-, NO2-, NO, U6+ (uranium), Fe3+, H+, etc. as final electron acceptors. Mammals use O2, and their respiration is therefore called aerobic respiration. Aerobic respiration happens in the inner mitochondrial membrane, which contains the relevant electron-transfering protein complexes. each of these complexes accepts electrons from a molecule and transfers them to a different compound, and the full assembly is therefore termed theelectron transport chain: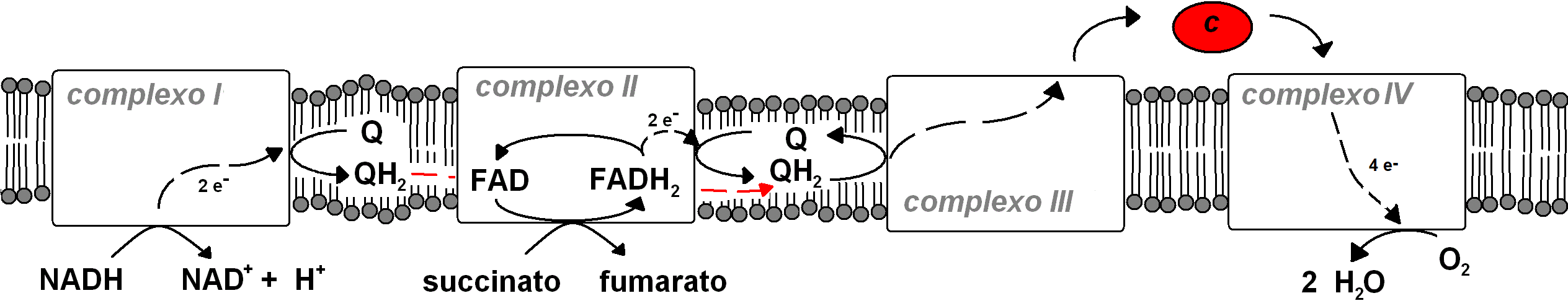
 In complexes I, III and IV , electron transfer releases enough energy to transfer H+ from the mitochondrial matrix to the intermembrane space. This causes an increase of H+ concentration (and electric potential) in the intermembrane s+ace, i.e. a larger chemical potential of H+ in the intermembrane space relative to the matrix. However, when we have two solutions with different concentrations on both sides of a membrane, solute tend to diffuse from the regions of higher chemical potential to areas of lower potential (for a neutral species, this is equivalent to moving from areas of higher concentration to areas of lower concentration).
In complexes I, III and IV , electron transfer releases enough energy to transfer H+ from the mitochondrial matrix to the intermembrane space. This causes an increase of H+ concentration (and electric potential) in the intermembrane s+ace, i.e. a larger chemical potential of H+ in the intermembrane space relative to the matrix. However, when we have two solutions with different concentrations on both sides of a membrane, solute tend to diffuse from the regions of higher chemical potential to areas of lower potential (for a neutral species, this is equivalent to moving from areas of higher concentration to areas of lower concentration).
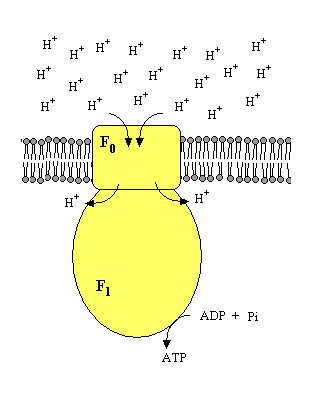
The inner mitochondrial membrane is not permeable to H+. Under normal conditions, the only way for protons to flow back to the matrix is through a special protein: ATP synthetase. This complex protein contains two major portions: an intermembrane proton channel (F0) coupled to a catalytic protein complex (F1) facing the mitochondrial matrix. The F1 portion contains several subunits with different functions, and converts the energy released by the return of protons to the matrix into chemical energy used to synthesize ATP from ADP and Pi.
NADH is unable to cross the mitochondrial membrane. There are therefore mechanisms to transfer electrons from NADH molecules produced in the cytoplasm during glycolisis to the electron transport chain. These are:
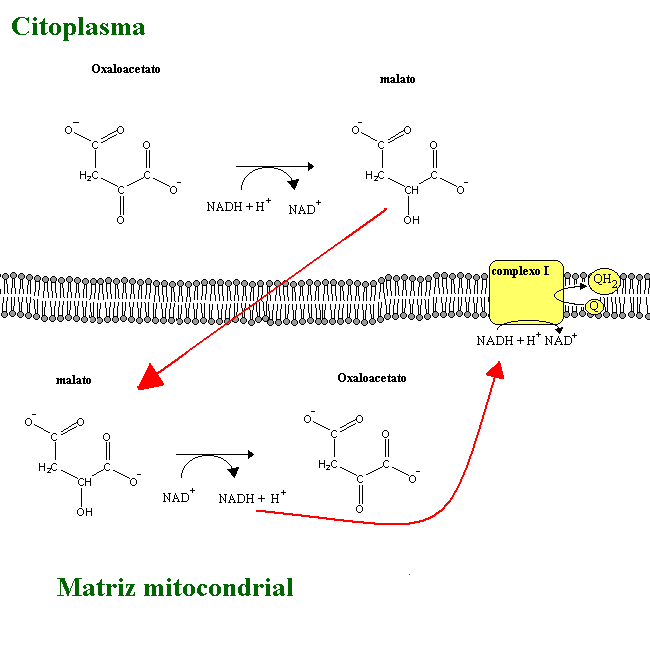
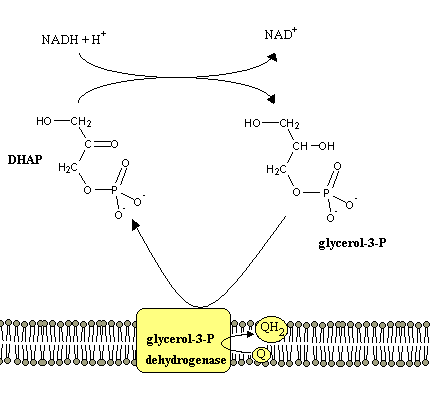
Mitochondrial respiration may occur without ATP production, as long as the released protons are able to return to the matrix without passing through the ATP synthetase. This can happen e.g. if ionophores (lipid-soluble molecules with the ability to transport ions) are added to the mitochondria. In brown adipose tissue, a special protein (thermogenin) forms a proton channel in the mitochondion inner membrane. The flow of protons back into the matrix through this protein instead of ATP synthetase is responsible for the heat generation characteristic of this tissue.
 |
Biochemistry,
by Donald Voet & Judith Voet An excellent text. It presents Biochemistry with frequent references to organic chemistry and biochemical logic. Highly reccommended for students of Biochemistry, Chemistry and Pharmaceutical Sciences. |
 |
Biochemistry,
Stryer A widely used classical text, frequently updated and re-issued. |
 |
Textbook
of Biochemistry with Clinical Correlations, Thomas Devlin Strongly advised to students in Nursing, Medicine, Dentistry, etc. Plenty of examples of application of biochemical knowledge to clinical cases. |
 |
Principles
of Biochemistry, Lehninger A widely used classical text, frequently updated and re-issued. |