|
| |

In contrast, water-soluble hormones cannot cross the membrane and bind to the external face of membrane-spanning proteins. In this instance, the conformational changes brought about on the receptor upon ligand binding trigger fast processes of activation (or deactivation) of proteins already present in the cell. These hormones act therefore much faster (in second to minutes). The tranduction of the hormonal signal may occur in different ways:
| opening ionic (Na+, K+, Cl-, Ca2+) channels. The influx of ions modifies the cell membrane potential. Ca2+ also binds to a specific protein (calmodulin) and activates it. Activated calmodulin may itself activate a wide array of other proteins present in the cell. | |
| phosphorylation of specific intracellular proteins by an intercellular domain of the receptor with protein kinase activity. Many proteins may exist in two forms, either phosphorylated or dephosphorylated, only one of which is physiologically active. Kinases therefore activate (or deactivate) proteins already present in the cell, leading to a very fast change on the amount (and kind) of active enzymes in the cell. | 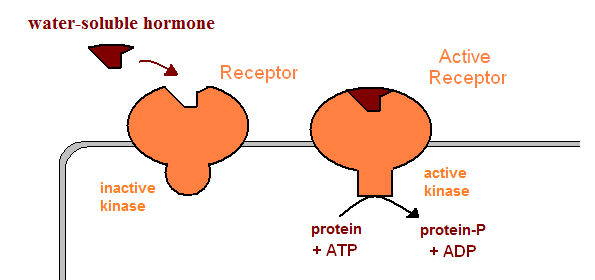 |
| activation of cytoplasmic kinases by the intracellular domain of the activated receptor | |
| activation of G-proteins. G-proteins contain three subunits, one of which binds GDP. When a G-protein binds an activated receptor, its GDP-binding subunits undergoes a subtle conformational change, and exchanges its GDP nucleotide by a GTP. The GTP-bound subunit can no longer bind the other subunits, and separates. This isolated GTP-bound subunit may the activate different signal transduction mechanisms: | 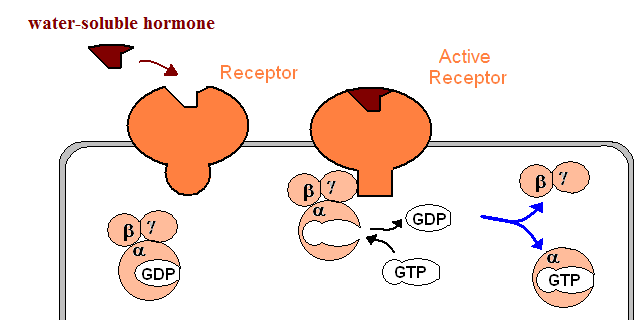 |
| activation of adenylyl (or guanylyl) cyclases which produce the second messengers cAMP (or cGMP). These second messengers activate specific kinases, which in turn activate (or deactivate) a wide array of enzymes. | 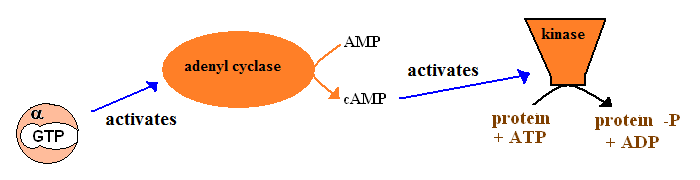 |
| activation of phospholipase C. This enzyme kydrolyzes a specific phospholipid, yielding two second messengers: inositol triphosphate (IP3) and a diacylglycerol (DAG). DAG activates the (membrane-bound) protein kinase C, and IP3 triggers Ca2+ release from the endoplasmic reticulum. | 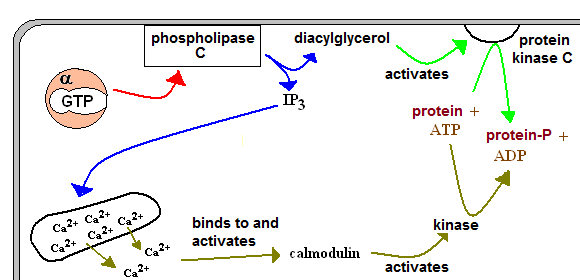 |
 | Human Physiology: The Mechanisms of Body function A good text for undergraduate students. |
 | Textbook of Medical Physiology A classic on Human Physiology. Excellent and detailed, it is invaluable for Nursing and Medicine students. |