REFERENCES:The role of hydrogen in the metabolism of Pyrococcus furiosus
P. furiosus is a strictly anaerobic Archaeon that grows near 100ºC by fermentation of carbohydrates through a modified Embden-Meyerhoff pathway, producing acetate, alanine, CO2 and H2 . Two soluble H2-producing enzymes are known in this organism: sulfhydrogenase I and sulfhydrogenase II . During metabolism of carbohydrates and amino acids, reducing equivalents are transferred to an iron-sulfur protein, ferredoxin. The oxidized form of ferredoxin must be regenerated in order to sustain the continuing operation of these metabolic pathways. However, the sulfhydrogenases from P. furiosus cannot use reduced ferredoxin as an electron donor, which prevents them from directly disposing of the reducing equivalents produced by the metabolism of P. furiosus. Instead, they are able to oxidize NADPH to NADP+ with concomitant production of H2. It was therefore suggested that these reducing equivalents may be transferred to NADP+ (thus generating NADPH) by a sulfide dehydrogenase, which can also function as a ferredoxin:NADP+ oxidoreductase . The produced NADPH will be used to reduce hydrogenase [1]. It has been shown that the reduction, catalyzed by enzymes, of protons to H2 by pyridine nucleotides is possible only when the partial pressure of H2 is kept below 0.1 kPa. P. furiosus is however able to grow under H2 accumulating conditions (pH2 > 30 kPa).
In order to understand the pathway of electron disposal in P. furiosus each of the enzymes involved (sulfide dehydrogenase and sulfhydrogenase) was purified and characterized. The physiological activities of these proteins in cell extracts were also investigated. The results of this study are succintly described below, and available in my PhD thesis.
SULFHYDROGENASE
Sulfhydrogenase revealed a very rich and intricate temperature-dependent redox chemistry. Although the enzyme has measurable H2-uptake activity at 45 ºC, analysis of the temperature-dependence of this activity suggests that the kinetic properties of the enzyme change markedly at 64 ºC [2] .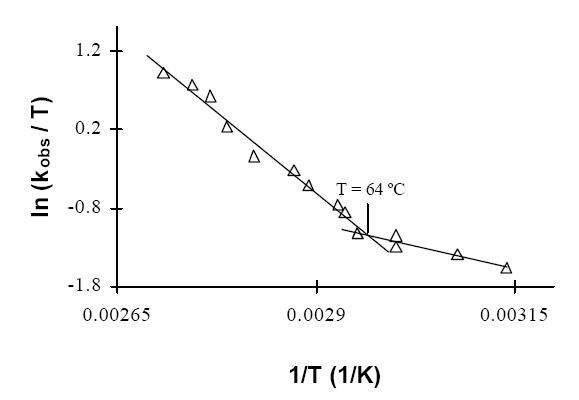
This may reflect a change of conformation of the protein or of the active site, e. g., the transition of the [NiFe] center from the "unready" to the "ready" state or a change of the redox potentials of the metal centers involved. Upon incubation of the oxidized sulfhydrogenase in the presence of H2 a remarkable increase in activity occurs. After activation the H2-evolution / H2-uptake ratio was observed to be less than unity [2], suggesting that the role of sulfhydrogenase in H2 evolution will not be as important as assumed in the model of Ma et al.[1] .
Incubation of anaerobically-oxidized sulfhydrogenase at 80 ºC revealed a complex process of temperature-induced self-reduction [3] . The identity of the internal reductant responsible for this process is not clear. Dithiothreitol seems to inhibit the self-reduction, suggesting the involvement of disulfide bridges in the process. This process has provided a very convenient way to generate different states of the [NiFe] active center for spectroscopic study.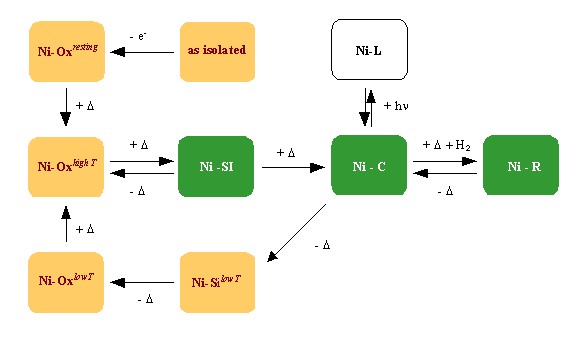
The temperature-induced activation (in orange) and catalytic cycle (in green) of sulfhydrogenase I. +D: anaerobic incubation at 80 ºC -D: anaerobic incubation at room temperatureMost of these states are heterogeneous, showing signals attributed to "ready" and "unready" hydrogenase. Their EPR spectra are also significantly more rhombic than the ones observed in mesophilic hydrogenases (cf. [4] and references therein). Several other interesting differences were also found. The Ni-C signal of P. furiosus sulfhydrogenase saturates much easier than the corresponding signals from Desulfovibrio hydrogenases and, though the Ni-C signal disappears upon illumination at low temperature as observed in regular hydrogenases, no new paramagnetic species (corresponding to Ni-L) were detected.
All of these data point to the existence of subtle differences in the electronic environment of the [NiFe] center of the P. furiosus enzyme. It is possible that some of these differences reflect an adaptation of the active site to the thermodynamic conditions under which it must operate.
The role of the detected [Fe-S] clusters is still not clear. Sequence analysis has indicated that probably more [Fe-S] clusters are present in the enzyme than have so far been examined by EPR. Whether these clusters are not detected due to very low redox potentials or to other unusual properties remains to be determined. The results of a redox titration at high temperature (PhD thesis, section 4.6) suggest that under some conditions one of the clusters cannot be reduced by dithionite, even though the solution potential is much lower than the redox potential of this cluster. The undetected clusters may have a similar behavior, e. g. kinetically hampered reduction due to inaccessibility from the solvent.
SULFIDE DEHYDROGENASE [5]
Sulfide dehydrogenase is a versatile flavo-iron-sulfur protein that catalyzes both the reduction of polysulfide by NADPH and of NADP+ by ferredoxin [1]. Reduction of NADP+ has been proposed to be a necessary step in the disposal of reducing equivalents as H2. The physiological role of sulfur reduction, however, is not clear.
A detailed study of the prosthetic groups of the sulfide dehydrogenase through sequence analysis and EPR-monitored redox titrations has provided a clearer picture of the composition of sulfide dehydrogenases. The protein was shown to carry three [Fe-S] clusters, each of them atypical in some way: a putative [2Fe-2S] cluster with novel Asp(Cys)3 coordination and with the combination of physico-chemical properties hitherto exclusively ascribed to Rieske-type clusters; an apparently regular [3Fe-4S] cluster with an unusually high reduction potential; and a [4Fe-4S] cluster with unusual relaxation properties and reduction properties. The redox potential of the flavin is consistent with the proposed functions as S0-reductase and ferredoxin:NADP+ oxidoreductase, but the role of the high potential [2Fe-2S] and [3Fe-4S] in these reactions is not obvious.
Each of the subunits of SuDH appears to be a member of a different class of iron-sulfur flavoproteins. The SudB subunit is very similar to the reductase+ferredoxin systems from plants and algae. The SudA subunit carries the basic pattern found in a small class of iron-sulfur flavoproteins with iron-sulfur clusters of unusual properties, and for which crystallographic data are not yet available. It is likely that structural and functional studies of these homologous proteins may shed light on the mechanism of action and physiological functions of sulfide dehydrogenase.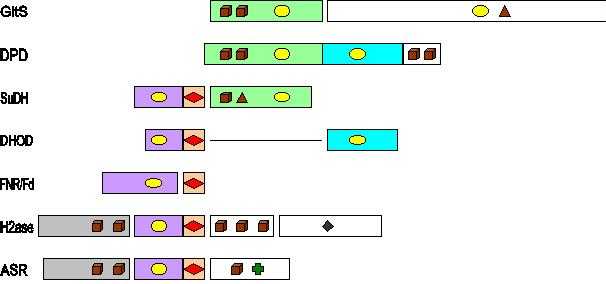
GltS, glutamate synthase (Azospirillum brasilense); DPD, dihydropyrimidine dehydrogenase (porcine liver); SuDH, sulfide dehydrogenase (P. furiosus); DHOD, dihydroorotate dehydrogenase (Lactococcus lactis); FNR, ferredoxin: NADP+ oxidoreductase (Spinach); Fd, ferredoxin (Spinach); H2ase, sulfhydrogenase (P. furiosus); ASR, assimilatory sulfite reductase (Salmonella typhimurium). Brown cubes stand for [4Fe-4S], brown triangles for [3Fe-4S], red diamonds for [2Fe-2S], yellow ellipses for FAD/FMN, a dark green diamond for the Ni-Fe dimer in hydrogenase, and a green cross for the siroheme in sulfite reductase.
THE PATHWAY OF HYDROGEN EVOLUTION FROM REDUCED FERREDOXIN [6]
The actual hydrogen production by cell extracts of P. furiosus is much higher than accounted for by the activities of the enzymes involved in the pathway proposed by Ma et al. [1]. Most of the ferredoxin-dependent H2-evolution is associated with the membrane.
The membrane-bound hydrogenase responsible for this activity was partially purified and characterized. It is a hydrogen-evolving hydrogenase (H2-evolution ca. 250 times greater than H2 uptake) virtually insensitive to CO. It can be inactivated by incubation with DCCD, which suggests the presence of proton-translocating segments in the protein, possibly in the MbhM subunit. The membrane-bound hydrogenase may thus couple H2 evolution to the formation of an electrochemical Na+ or H+ gradient, as proposed for similar hydrogenases from M. barkeri and R. rubrum .
The protein is predicted to harbor three [Fe-S] clusters. EPR measurements show a signal originating from at least two interacting clusters with a redox potential ca. –0.33 V (at room temperature). No EPR signals originating from the [NiFe] active site could be found. Even at room temperature the membrane-bound hydrogenase is quite active, and redox titrations are hampered by the diversion of reducing equivalents to proton reduction. In order to circumvent this problem it might be worthwhile to inhibit the enzyme with DCCD prior to a redox titration, since in principle DCCD will not interfere with the active site, but only with the MbhM subunit.
The thermodynamics of the process are also not fully understood. It is known that the redox potential of ferredoxin at high (i.e physiological) temperature is approximately equal to that of the proton/H2 couple, but this value is probably less relevant for the bioenergetics because ferredoxin is an electron-transfer intermediate. Presumably more important values (presently not available) will be the steady-state concentrations of the ultimate electron donors, glyceraldehyde-3-phosphate and pyruvate and their oxidation products. This knowledge would allow the estimation of the magnitude of the electrochemical gradient that might be generated by the membrane-bound hydrogenase.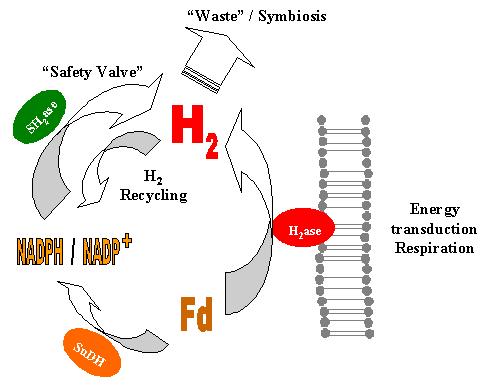
The results obtained nonetheless allow the formulation of a novel model of the hydrogen metabolism in P. furiosus. Reduced ferredoxin produced by the catabolism transfers its electrons to the membrane-bound hydrogenase, which reduces protons to H2. If the DG of the process is favorable, concomitant H+ or Na+ translocation through the membrane will occur, allowing the formation of a gradient to be used in ATP synthesis or active transport. When the flow of reducing equivalents from ferredoxin is greater than can be handled efficiently by the membrane-bound hydrogenase, the pathway proposed by Ma et al. will become very relevant to the cell, allowing the disposal of the extra electrons. The H2 formed can be used by the soluble hydrogenase to reduce NADP+, thus providing an extra source of NADPH for anabolic processes.
INSIGHTS FROM SEQUENCE COMPARISONS
The recently available genome information shows the presence of another operon in P. furiosus that may code for a membrane-bound hydrogenase. The operon contains fourteen genes, each of them preceded by a ribosome-binding site. This suggests that this operon is effectively transcribed under at least some conditions. This putative hydrogenase contains an unusual binding motif for the [NiFe] cluster, which appears to be m-oxo instead of m-sulfo-bridged. Its electronic properties can thus be expected to deviate from those observed in the other hydrogenases.
Sequence comparisons between several hydrogenases have allowed the description of specific signatures that can be used to selectively distinguish these membrane-bound hydrogenases from each other and from the very similar NADH: ubiquinone oxidoreductases. Similar comparisons between sulfhydrogenases, F420-reducing-, F420-non-reducing- and NAD-linked hydrogenases have suggested the possibility that some of the clusters in the small subunits of sulfhydrogenases and F420-non-reducing-hydrogenases may have pH-dependent potentials, allowing a more precise regulation of activity according to the physiological conditions. The validity of this proposal should be tested by redox titrations performed at different pHs.
[1] Hydrogen production from pyruvate by enzymes purified from the hyperthermophilic archaeon, Pyrococcus furiosus: A key role for NADPH.K. Ma, Z.H. Zhou and M.W.W. Adams. FEMS Microbiol. Lett. (1994) 122: 245-250.
[2] Effects of temperature on the electron transfer between Pyrococcus furiosus hydrogenase and its redox partners. P.J. Silva, M.J. Amorim, P.-L. Hagedoorn, H. Wassink, H. Haaker and W.R. Hagen. Journal of Inorganic Biochemistry (1999) 74, 297.
[3] On the prosthetic groups of the NiFe sulfhydrogenase from Pyrococcus furiosus: topology, structure, and temperature-dependent redox chemistry. Pedro J. Silva, Baltazar de Castro and Wilfred R. Hagen. Journal of Biological Inorganic Chemistry (1999) 4, 284-291.
[4] Nickel hydrogenases: in search of the active site. Simon P. Albracht. Biochim Biophys Acta (1994) 1188, 167-204.
[5] Novel
structure and redox chemistry of the prosthetic groups of the iron-sulfur
flavoprotein sulfide dehydrogenase from Pyrococcus furiosus; evidence
for a [2Fe-2S] cluster with Asp(Cys)3 ligands. W.R.
Hagen, P.J. Silva, M.A. Amorim, P.-L. Hagedoorn, H. Wassink, H. Haaker
and F.T. Robb. Journal of Biological Inorganic Chemistry (2000)
5, 527-534.
[6] Enzymes
of hydrogen metabolism in Pyrococcus furiosus. Pedro J.
Silva, Eyke C. D. van den Ban, Hans Wassink, Huub Haaker, Baltazar de Castro,
Frank T. Robb and Wilfred R. Hagen, European Journal of Biochemistry
(2000)
267, 6541-6551.