REFERENCES:Previous Home
The pathway of hydrogen evolution from reduced ferredoxin in Pyrococcus furiosus [1]
The actual hydrogen production by cell extracts of P. furiosus is much higher than accounted for by the activities of the enzymes involved in the pathway proposed by Ma et al. [2]. Most of the ferredoxin-dependent H2-evolution is associated with the membrane.
The membrane-bound hydrogenase responsible for this activity was partially purified and characterized [1, my PhD thesis]. It is a hydrogen-evolving hydrogenase (H2-evolution ca. 250 times greater than H2 uptake) virtually insensitive to CO. It can be inactivated by incubation with DCCD, which suggests the presence of proton-translocating segments in the protein, possibly in the MbhM subunit. The membrane-bound hydrogenase may thus couple H2 evolution to the formation of an electrochemical Na+ or H+ gradient, as proposed for similar hydrogenases from M. barkeri and R. rubrum .
The protein is predicted to harbor three [Fe-S] clusters. EPR measurements show a signal originating from at least two interacting clusters with a redox potential ca. –0.33 V (at room temperature). No EPR signals originating from the [NiFe] active site could be found. Even at room temperature the membrane-bound hydrogenase is quite active, and redox titrations are hampered by the diversion of reducing equivalents to proton reduction. In order to circumvent this problem it might be worthwhile to inhibit the enzyme with DCCD prior to a redox titration, since in principle DCCD will not interfere with the active site, but only with the MbhM subunit.
The thermodynamics of the process are also not fully understood. It is known that the redox potential of ferredoxin at high (i.e physiological) temperature is approximately equal to that of the proton/H2 couple, but this value is probably less relevant for the bioenergetics because ferredoxin is an electron-transfer intermediate. Presumably more important values (presently not available) will be the steady-state concentrations of the ultimate electron donors, glyceraldehyde-3-phosphate and pyruvate and their oxidation products. This knowledge would allow the estimation of the magnitude of the electrochemical gradient that might be generated by the membrane-bound hydrogenase.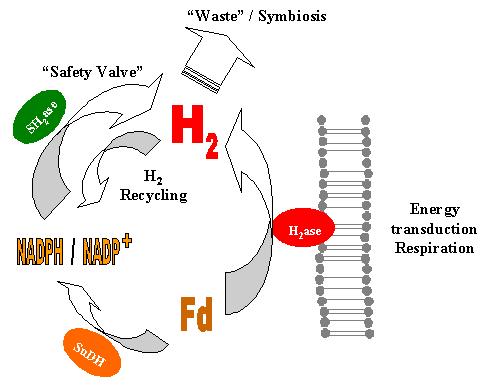
The results obtained nonetheless allow the formulation of a novel model of the hydrogen metabolism in P. furiosus [1]. Reduced ferredoxin produced by the catabolism transfers its electrons to the membrane-bound hydrogenase, which reduces protons to H2. If the DG of the process is favorable, concomitant H+ or Na+ translocation through the membrane will occur, allowing the formation of a gradient to be used in ATP synthesis or active transport. When the flow of reducing equivalents from ferredoxin is greater than can be handled efficiently by the membrane-bound hydrogenase, the pathway proposed by Ma et al. will become very relevant to the cell, allowing the disposal of the extra electrons. The H2 formed can be used by the soluble hydrogenase to reduce NADP+, thus providing an extra source of NADPH for anabolic processes.
INSIGHTS FROM SEQUENCE COMPARISONS
The recently available genome information shows the presence of another operon in P. furiosus that may code for a membrane-bound hydrogenase. The operon contains fourteen genes, each of them preceded by a ribosome-binding site. This suggests that this operon is effectively transcribed under at least some conditions. This putative hydrogenase contains an unusual binding motif for the [NiFe] cluster, which appears to be m-oxo instead of m-sulfo-bridged. Its electronic properties can thus be expected to deviate from those observed in the other hydrogenases.
Sequence comparisons between several hydrogenases have allowed the description of specific signatures that can be used to selectively distinguish these membrane-bound hydrogenases from each other and from the very similar NADH: ubiquinone oxidoreductases. Similar comparisons between sulfhydrogenases, F420-reducing-, F420-non-reducing- and NAD-linked hydrogenases have suggested the possibility that some of the clusters in the small subunits of sulfhydrogenases and F420-non-reducing-hydrogenases may have pH-dependent potentials, allowing a more precise regulation of activity according to the physiological conditions. The validity of this proposal should be tested by redox titrations performed at different pHs.
[1] Enzymes of hydrogen metabolism in Pyrococcus furiosus. Pedro J. Silva, Eyke C. D. van den Ban, Hans Wassink, Huub Haaker, Baltazar de Castro, Frank T. Robb and Wilfred R. Hagen, European Journal of Biochemistry (2000) 267, 6541-6551.
[2] Hydrogen production from pyruvate by enzymes purified from the hyperthermophilic archaeon, Pyrococcus furiosus: A key role for NADPH.K. Ma, Z.H. Zhou and M.W.W. Adams. FEMS Microbiol. Lett. (1994) 122: 245-250.