Professor Auxiliar, Universidade Fernando Pessoa
| Other metabolic pathways: |
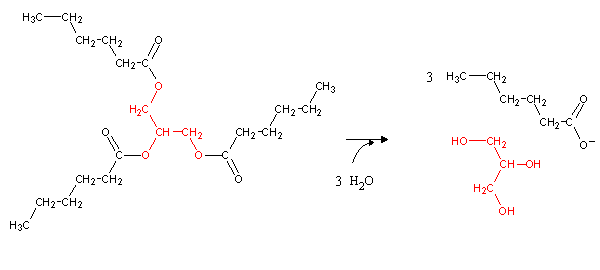
Most energy reserves in the body are stored as triacylglycerides, which can be hydrolyzed to glycerol and fatty acids through the action of lipases:
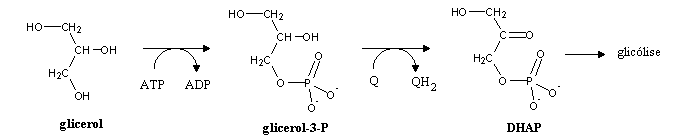
Glycerol can be metabolized by glycolysis upon oxidation (in the outer face of the inner mitochodrial membrane) to dihydroxyacetone phosphate. Both electrons are taken up by ubiquinone (Q), and are fed into the electron transport chain.
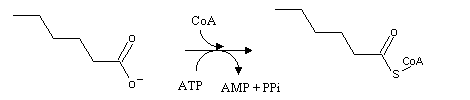
Fatty acids follow a different pathway: b-oxidation, which takes place in the mitochondrion. Before entering the mitochondrion, fatty acids must be activated. The activation reaction happens in the cytoplasm, and it consists on the transformation of the fatty acid into its acyl-Coa derivative. As we have seen in the citric acid cycle, thioester bonds are very energetic. Therefore, an ATP gets hydrolyzed (to AMP, which is equivalent to the hydrolysis of 2 ATP to 2 ADP) in the process.
The mithochondrial inner membrane is impermeable to acyl-CoAs. In order to get inside, these will react with a "special" aminoacid, carnitine, releasing CoA. Sterified carnitine is transported into the mitochondial matrix by a specific membrane-bound transport complex. Inside the mitochondrion, carnitine transfers the acyl group to another CoA molecule. Free carnitine returns to the cytoplasm through the same transporter complx. In this process, no net CoA transport into the mitochondrion occurs: separate cytoplasmic and mitochondrial CoA pools are kept .
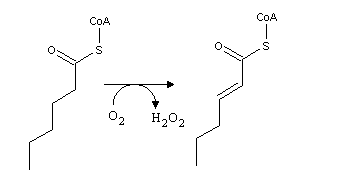
Fatty acids b-oxidation is a cyle composed of three consecutive reactions, which are identical to the last part of the citric acid cycle: dehydrogenation, hydration of the newly formed C=C double bond, and oxidation of the alcohol to a ketone:
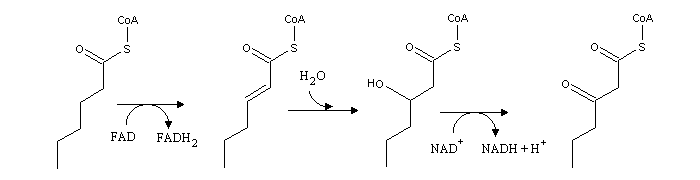
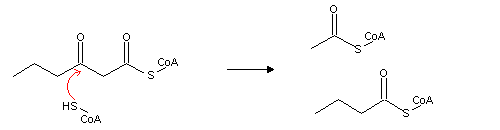
From the product of these reactions, the enzyme thiolase releases acetyl-CoA and an acyl-CoA with two carbon atoms less than the original acyl-CoA.
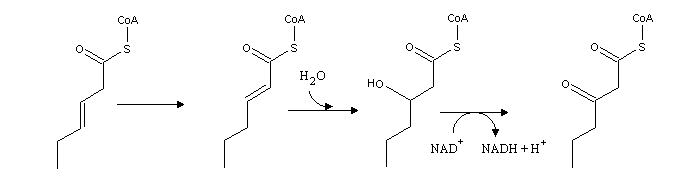
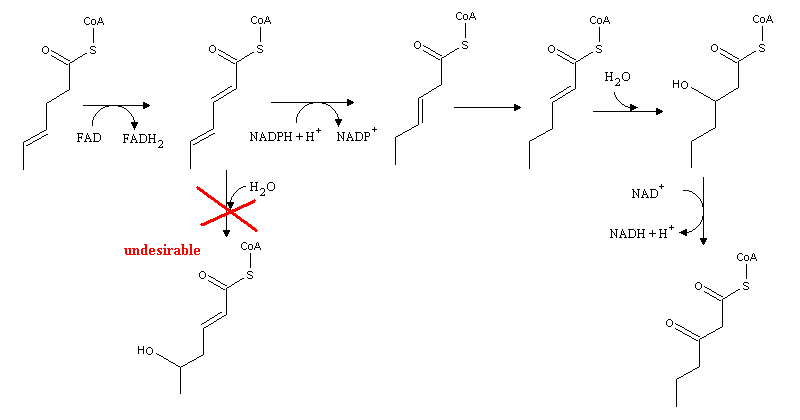
Insaturated fatty acids follow a similar pathway, although new enzymes are needed to deal with the C=C double bonds. If a double bond lies on an odd C atom, Δ3, Δ2-enoyl-CoA isomerase is needed: this enzyme transfers the double bond from C3 to C2, thereby allowing β-oxidation. In this β-oxidation cycle no FADH2 is formed.
When the double bond lies on an even-numbered carbon, 2,4-dienoyl-CoA reductase is needed, since the presence of cunjugated double bonds makes hydration more favorable on carbon 4, rather than on the "right" carbon (2). 2,4-dienoyl-CoA reductase uses two electrons from NADPH to reduce the Δ4, Δ2 system, and form a single double bond on carbon 3. Oxidation then follws the same procedure used for fatty acids bearing a double bond on an odd-numbered carbon..
Succesive rouds of the cycle eventually lead to the total degradation of even-chain fatty acids in acetyl-CoA, which can be completely oxidized to CO2 through the citric acid cycle: even-chain fatty acids cannot be used for net synthesis of oxaloacetate, and therefore are not a substrate for gluconeogenesis. In the last round of b-oxidation, odd-chain fatty acids
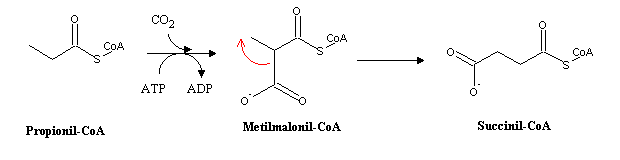
Succinyl-CoA is an intermediate in the citric acid cycle and also a precursor of heme biossynthesis. A vitamin B12 deficiency therefore impairs the ability to synthesize heme and may eventually lead to the onset of pernicious anemia . This disease is usually caused by the lack of ability to retrieve cobalamin from the nutrients in the stomach, and is observed in predisposed individuals in old age. Before modern methos of cobalamin production, treatment of this disease consisted in the daily uptake of large amounts of raw liver, which is a cery good reservoir of this heat-labile vitamin. The almost exclusive onset of the disease in old patients is a consequence of the presence in our own liver of a B12 stock enough for about 3-5 years, so that the effects of an impairment of its absorption will be very delayed.
Succinyl-CoA can be oxidized by the citric acid cycle to malate, which after moving into the cytoplasm can be used in gluconeogenesis. In the cytoplasm, malate can also be decarboxylated to pyruvate by the malic enzyme, with cincommitant NADPH production:
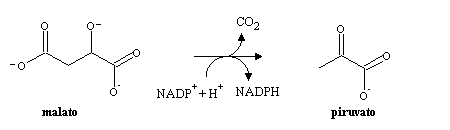
Pyruvate formed in this reaction can enter the mitochondrion and be oxidized completely to CO2 by the citric acid cycle.
Peroxisomes are small organelles where the initial steps of b-oxidation of very long chain fatty acids occur. The major differences between mitochondrial and peroxisomal b-oxidation are:
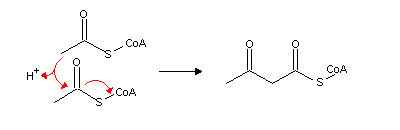
Much of the acetyl-CoA produced by fatty acid b-oxidation in liver mitochodria is converted in acetoacetate and b-hydroxybutyrate (also known as ketone bodies). These molecules can be used by heart and skeletal muscle to produce energy. Brain, which usually depends on glucose as sole energy source, can also use ketone bodies during a long fasting period (larger than two or three days). Ketogenesis (ketone bodies synthesis) begins with the condensation of two acetyl-CoA molecules to form acetoacetyl-CoA:
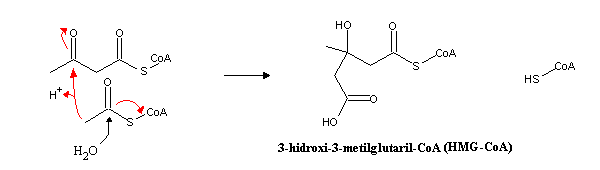
Condensation of another acetyl-CoA molecule yields 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA). The basic mechanism of this reaction is identical to the condensation of oxaloacetate with acetyl-CoA to produce citrate, the first step in the citric acid cycle.
HMG-CoA is afterwards cleaved in acetoacetate and acetyl-CoA:
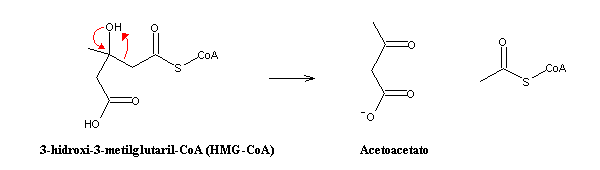
Acetoacetate moves into the bloodstream and gets distributed to the tissues. Once absorbed, it reacts (in mitochondria) with succinyl-CoA, yielding succinate and acetoacetyl-CoA, which can be cleaved by thiolase into two molecules of acetyl-CoA.
When acetyl-CoA is abundant, liver and adipose tissue synthesize fatty acids. The syntheis pathway is quite similar to the reverse of b-oxidation, but presents several imporatant differences:
Fatty acids synthesis uses acetyl-CoA as main substrate. However, since the process is quite endergonic acetyl-CoA must be activated, which happens through carboxylation. Like other carboxylases (e.g., those of pyruvate or propionyl-CoA), Acetyl-CoA carboxilase uses biotin as a prosthetic group.
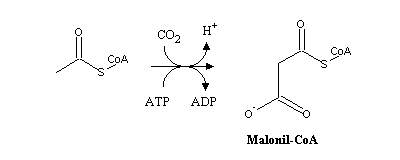
Malonyl-CoA is afterwards transferred to the acyl carrier protein (ACP), yielding malonyl-ACP, which will condense with acetyl-ACP (sinthesized likewise from acetyl-CoA).
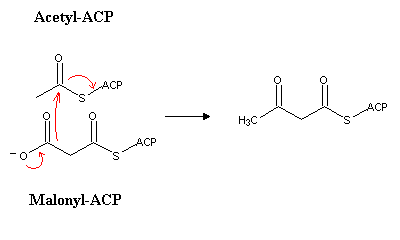
In animals, every step of palmitic acid (the 16-carbon saturated fatty acid) synthesis is catalyzed by fatty acid synthase, a very large enzyme with multiple enzymatic activities. Butiryl-ACP produced in the first reaction will be transformed in butyl-ACP (the 4-carbon acyl-ACP). The reaction sequnce is the reverse of b-oxidation, i.e., reduction, dehydration and hydrogenation:
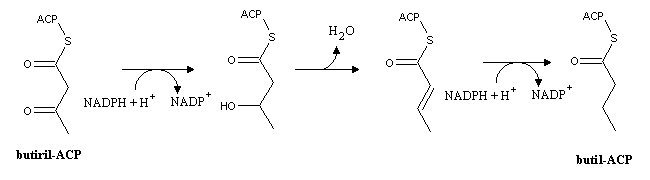
Butyl-ACP can afterwards condense with another malonyl-ACP molecule. After seven rounds of this cycle palmitoyl-ACP is produced. Palmitoyl-ACP hydrolysis yields palmitic acid. The stoichiometry of palmitic acid synthesis is therefore:
Acetyl-CoA + 7 Malonyl-CoA + 14 NADPH + 7 H+ ---> palmitic acid + 7 CO2 + 14 NADP+ + 8 CoA + 6 H2O
Longer (or unsaturated) fatty acids are produced from palmitic acid by elongases and desaturases.
Fatty acid synthesis happens in the cytoplasm, but acetyl-CoA is produced in the mitochondrion. Therefore acetyl-CoA must cross the inner mitochondrial membrane before it can be used in fatty acid synthesis. This is performed by the citrate shuttle: citrate is formed in the mitochondrion by condensing acetyl-CoA with oxaloacetate and diffuses through the membrane into the cytoplasm, where it gets cleaved by citrate-lyase into acetyl-CoA and oxaloacetate, whic, upon reduction to malate, can return to the mitochondrial matrix. Malate can also be used to produce part of the NADPH needed for fatty acid synthesis, through the action of the malic enzyme. The remainder of the NADPH needed for fatty acid synthesis must be produced by the pentose phosphate pathway.
 |
Biochemistry,
by Donald Voet & Judith Voet An excellent text. It presents Biochemistry with frequent references to organic chemistry and biochemical logic. Highly reccommended for students of Biochemistry, Chemistry and Pharmaceutical Sciences. |
 |
Biochemistry,
Stryer A widely used classical text, frequently updated and re-issued. |
 |
Textbook
of Biochemistry with Clinical Correlations, Thomas Devlin Strongly advised to students in Nursing, Medicine, Dentistry, etc. Plenty of examples of application of biochemical knowledge to clinical cases. |
 |
Principles
of Biochemistry, Lehninger A widely used classical text, frequently updated and re-issued. |