Associate Professor, Universidade Fernando Pessoa
| Other metabolic pathways: |
The human body has two main ways to keep constant blood glucose levels between meals: glycogen degradation and gluconeogenesis. Gluconeogenesis is the synthesis of glucose from other organic compounds (pyruvate, succinate, lactate, oxaloacetate, etc. Most of the reactions involved are quite similar to the reverse of glycolysis. Indeed, almost all reactions in glycolyis are readily reversible under physiological conditions. The three exceptions are the reactions catalyzed by :
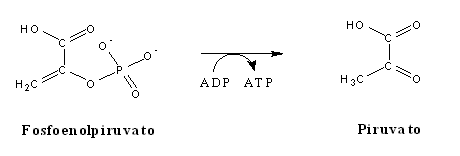
In gluconeogenesis, every one of these steps is replaced by thermodinamically favorable reactions. Among these three reactions, phosphoenolpyruvate synthesis from pyruvate is the most energy-demanding, since its DG is rather positive. In order to overcome this thermodynamic barrier, the reaction will be coupled to a decarboxylation, a strategy often used by the cell to displace an equilibrium towards the formation of products, as it will also be observed in several reactions in the citric acid cycle. Since both pyruvate and phosphoenolpyruvate(PEP) are three-carbon compounds, pyruvate must be carboxylated to a four-carbon compound, oxaloacetate (OAA), before such a decarboxylation can happen. The enzyme responsible for pyruvate carboxylation (pyruvate carboxylase) is present inside the mithocondrial matrix, and contains biotin, a CO2-activating cofactor. The energy required for the carboxylation comes from from the hydrolysis of ATP. Oxaloacetate decarboxylation releases the energy needed to enable C2 phosphorylation by GTP, yielding phosphoenolpyruvate (in a reaction catalyzed bynuma phosphoenolpyruvate carboxykinase - PEPCK).
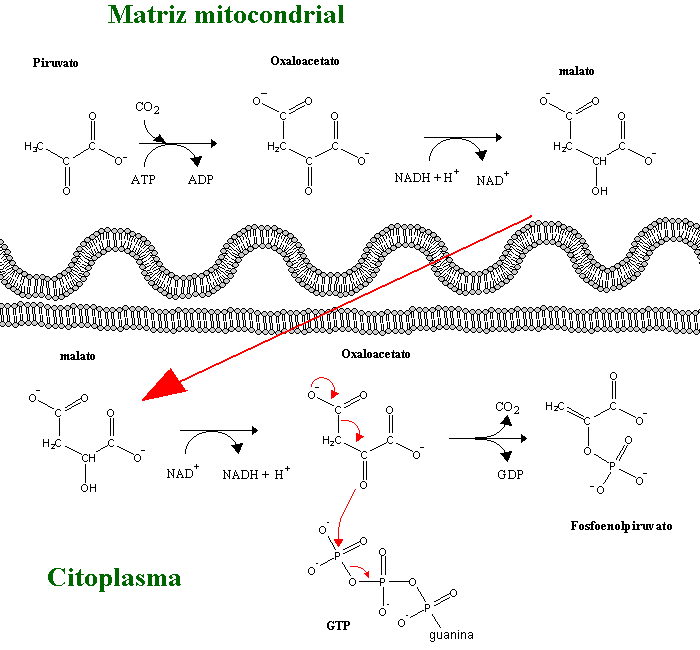
Oxaloacetate produced by the pruvate carboxylase cannot cross the mithochodrial membrane. It can only leave the mithochondrion after conversion to malate or aspartate. The choice of the process depens on the availability of cytoplasmic NADH (needed for gluconeogenesis). If there is enough NADH in th cytoplasm (e.g. when lactate is being used as gluconeogenic substrate) oxaloacetate will be transaminated to aspartate. Otherwise, OAA will be reduced to malate in the mithochondrial matrix. The mithochondrial membrane is permeable to malate, which moves into the cytoplasm, where it can be oxidized to oxaloacetate with concommitant production of NADH. Oxaloacetate can then be decarboxylated to PEP by the cytoplasmic PEPCK. Some tissues also contain a mithochondrial PEPCK.
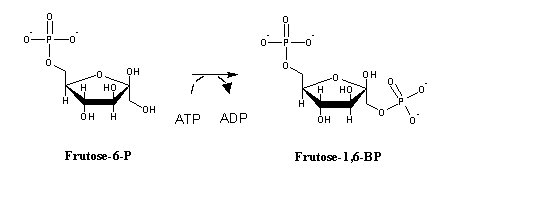
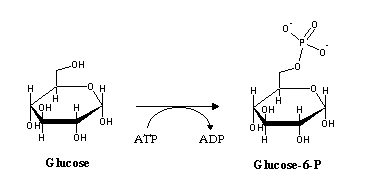
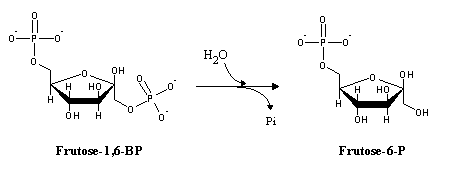
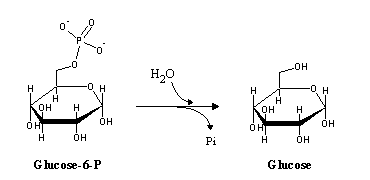
In gluconeogenesis, the reactions catalyzed by phosphofructokinase and hexokinase are replaced by hydrolytic reactions. Instead of phosphorylating ADP to ATP (the exact reverse of glycolysis, yet thermodynamically not favorable under physiological conditions), phosphate is released by hydrolysis:
 |
Biochemistry,
by Donald Voet & Judith Voet An excellent text. It presents Biochemistry with frequent references to organic chemistry and biochemical logic. Highly reccommended for students of Biochemistry, Chemistry and Pharmaceutical Sciences. |
 |
Biochemistry,
Stryer A widely used classical text, frequently updated and re-issued. |
 |
Textbook
of Biochemistry with Clinical Correlations, Thomas Devlin Strongly advised to students in Nursing, Medicine, Dentistry, etc. Plenty of examples of application of biochemical knowledge to clinical cases. |
 |
Principles
of Biochemistry, Lehninger A widely used classical text, frequently updated and re-issued. |