Associate Professor, Universidade Fernando Pessoa
| Other metabolic pathways: |
Pyruvate produced by glycolysis still contains a lot of reducing power (check each of its carbon atoms' oxidation state and compare it with carbon's oxidation state in CO2). This reducing power will be harnessed by the cell through the citric acid cycle. First, pyruvate is decarboxylated to acetyl-CoA, an activated form of acetate (CH3COO-)
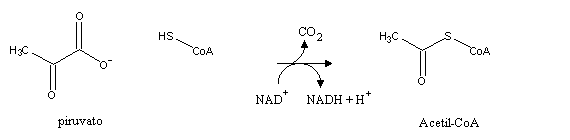
This reaction is catalyzed by pyruvate dehydrogenase, a very complex enzyme with several cofactors: lipoamide, FAD, coenzyme A. Thioester bond (S-C=O) hydrolysisis very exergonic, and therfore its formation demands energy. That energy comes from pyruvate decarboxylation (pyruvate contains three carbon atoms, and the acetyl portion of acetyl-CoA only contains two: the carboxylate group left as CO2). Energy from decarboxylations is often used bt the cell to push an equilibrium towards product formation, as can be seen in several reactions in the citric acid cycle and gluconeogenesis.
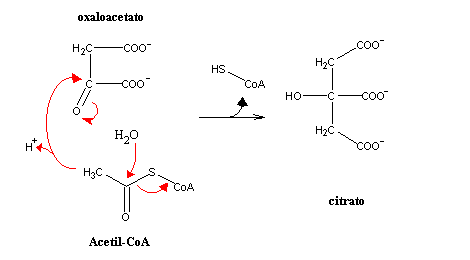
In the first reaction of the citric acid cycle, acetyl-CoA attacks oxaloacetate, yielding citrate, in an aldol addition. Thioester hydrolysis helps to displace equilibrium towards product formation:
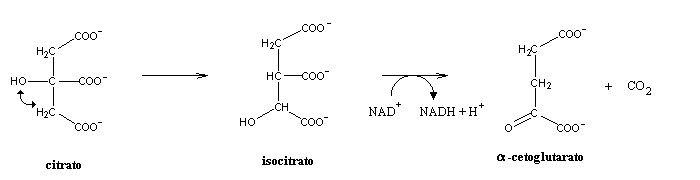
Citrate is then isomerized to isocitrate, which is then decarboxylated to a-ketoglutarate. If citrate had not been isomerized to isocitrate, this decarboxylation would yield a branched carbon compound, much harder to metabolize.
 a-ketoglutarate
is a a-ketoacid, i.e., it contains a carbonyl group adjacent to a carboxylic acid. We can predict that it will react like pyruvate, i.e.,
that its decarboxylation may yield enough energy to enable the formation of a thioester bond with coenzyme A. And this indeed occurs... The enzyme involved (a-ketoglutarate dehydrogenase), is quite similar to pyruvate dehydrogenase in composition, cofactors and mechanism.
a-ketoglutarate
is a a-ketoacid, i.e., it contains a carbonyl group adjacent to a carboxylic acid. We can predict that it will react like pyruvate, i.e.,
that its decarboxylation may yield enough energy to enable the formation of a thioester bond with coenzyme A. And this indeed occurs... The enzyme involved (a-ketoglutarate dehydrogenase), is quite similar to pyruvate dehydrogenase in composition, cofactors and mechanism.
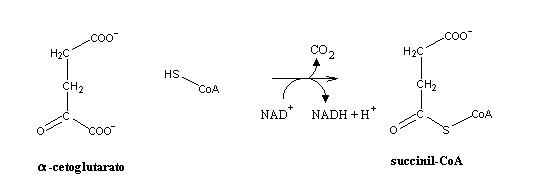
Like every thioester bond, the one present in succinyl-CoA is quite energetic. Its hydrolysis will be the only step in the citric acid cycle where direct production of ATP (or equivalent) occurs.
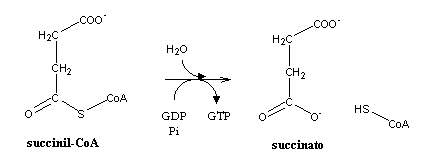
Like oxaloacetate, succinate is a four-carbon product. The last reactions of the citric acid cycle will regenerat oxaloacetate from succinate. Succinate is first oxidized to fumarate, by the succinate dehydrogenase complex (also known as complex II), which is present in the amtrix side of the inner mitochondrial membrane. The redox potential of the oxidation of a C-C single bond to a C=C double bond (alkanes to alkenes) is too high to enable the involved elevtrons to be accepted by NAD+ (E0=-320 mV). the cell will terefore use FAD (E0= 0 mV) as electron acceptor. Fumarate hydration yields malate, which can be oxidized to oxaloacetate, thus closing the cycle.A similar sequence of reactions happens in fatty acids b-oxidation.
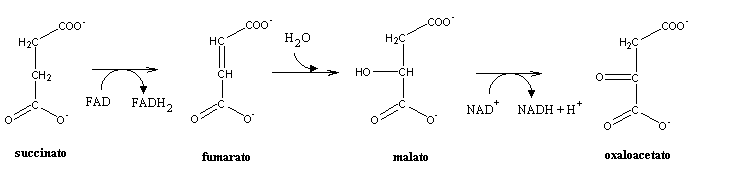
The end result of the citric acid cycle is therefore:
Acetyl-CoA + oxaloacetate + 3 NAD+ + GDP + Pi +FAD --> oxaloacetate + 2 CO2 + FADH2 + 3 NADH + 3 H+ + GTP
 |
Biochemistry,
by Donald Voet & Judith Voet An excellent text. It presents Biochemistry with frequent references to organic chemistry and biochemical logic. Highly reccommended for students of Biochemistry, Chemistry and Pharmaceutical Sciences. |
 |
Biochemistry,
Stryer A widely used classical text, frequently updated and re-issued. |
 |
Textbook
of Biochemistry with Clinical Correlations, Thomas Devlin Strongly advised to students in Nursing, Medicine, Dentistry, etc. Plenty of examples of application of biochemical knowledge to clinical cases. |
 |
Principles
of Biochemistry, Lehninger A widely used classical text, frequently updated and re-issued. |