Associate Professor, Universidade Fernando Pessoa
| Other metabolic pathways: |
Blood glucose levels are kept at approximately constant levels around 4-5 mM. Glucose enters cells by facilitated diffusion. Since this process does not allow the cell to contain glucose at a higher concentration than the one present in the bloodstream, the cell (through the enxyme hexokinase) chemically modifies glucose by phosphorylation:
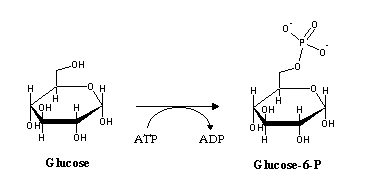
Since the cell membrane is impermeable to glucose-6-phosphate, this process effectively "traps" glucose inside the cell, allowing the recovery of more glucose from the bloodstream. Glucose-6-phosphate will be used in glycogen synthesis (a storage form of glucose) , production of other carbon compounds by the pentose-phosphate pathway, or degraded in order to produce energy- glycolysis.
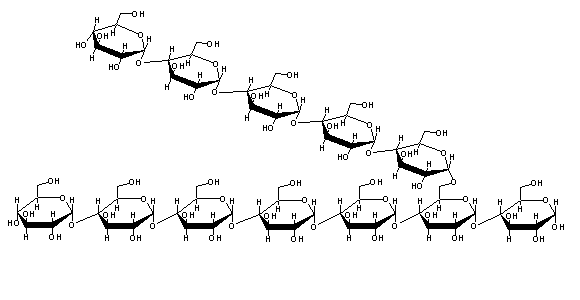
Large ammounts of glucose-6-P inside the cell cause and increase of the osmotic pressure. In these conditions, water will tend to flow into the cell, increasing its colume and (eventually) lysing it. In order to prevent this, the cell stores glucose-6-P as a polymer: glycogen. Glycogen is a sparsely soluble (and therefore osmotically inactive) branched polyssacharide, composed of glucose monomers joined through glycosidic bonds of the type a-1,4 and a-1,6 (in branching points) :
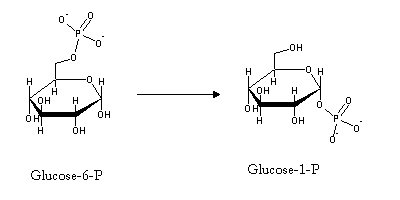
Addition of glucose-1-P to the 4' carbon of a glycogen chain is not favored thermodinamically, since the phosphate transfer potential of C-O-P bonds is quite low. Glucose-1-P will therefore be activated, i.e., transformed into a species with high phosphate transfer potential. This is accomplished by reaction with uridine triphosphate(UTP, an analog of ATP, with uridine replacing adenine).
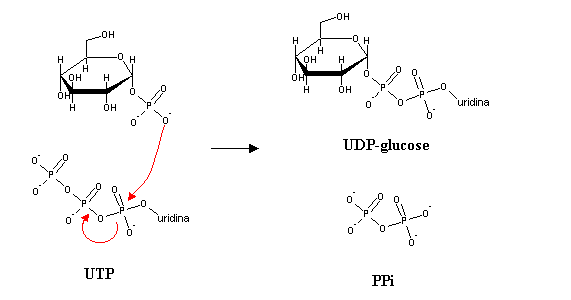
By itself, this reaction seems not to be thermodynamically favourable. However, pyrophosphate (PPi) released in this reaction can be hydrolyzed by the ubiquitous enzyme pyrophosphatase, in a very exergonic reaction. Removal of PPi pushes the equilibrium towards the formation of UDP-glucose, which illustrates the general principle that a very exergonic reaction can be coupled to an otherwise unfavourable reaction in order to make it spontaneous.
UDP-glucose has a high phosphate transfer potential, and this allows it to donate glucose to the 4' end of a glycogen chain, in a reaction catalyzed by glycogen synthase:
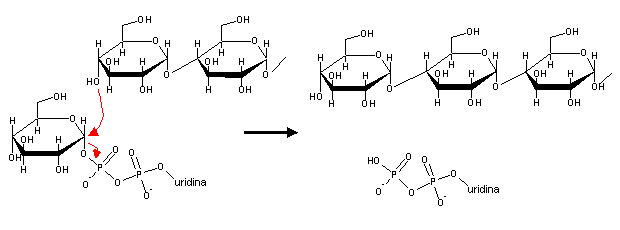
Glycogen synthase can only add glucose to pre-existent glycogen chains,i.e, it is unable to start the synthesis of a new glycogen molecule. Glycogen synthesis is started by the addition oa a glucose molecule to a tyrosine residue present in the active site of a protein called glycogenin. After addition of around seven more glucose molecules, the new glycogen chain is ready to be acted upon by glycogen synthase
Branching points are created by a "branching enzyme". This enzyme acts upon linear stretches of glycogen with at least 11 glucose molecules. Branching enzyme (amylo(1,4 -->1,6)-transglycosylase) transfers 7 glucose molecules-long terminal segments of glycogen to the OH group of carbon 6 of a glucose reidue (in the same or in another chain). Branching points must be at least 4 glucose molecules apart from each other.
Glycogen degradationGlycogen is degraded by the sequential action of three enzymes:
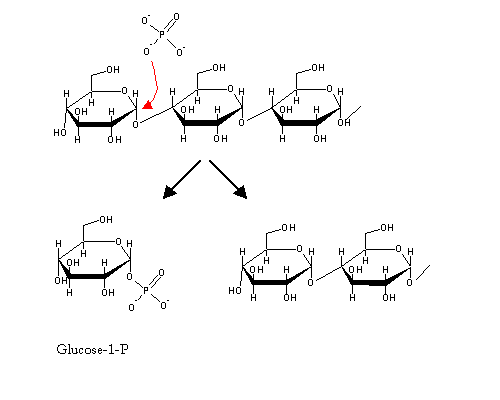
A glycogen molecule with branches of only four glucose molecules ("limit-dextrin") cannot be further degraded by glycogen phosphorylase alone. It needs another enzyme:
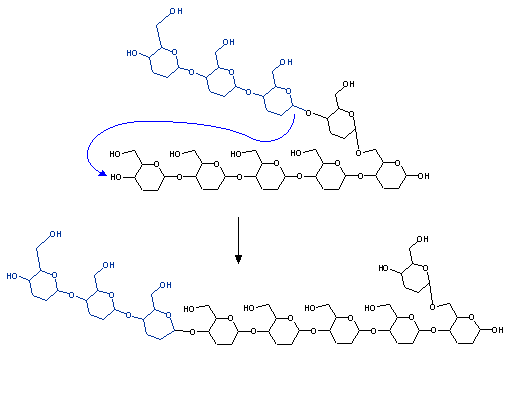
Glycogen phosphorylase is much faster than the debranching enzyme, and therefore the outer branches of glycogen are degraded bery rapidly in muscle when much energy is needed. Glycogen degradation beyond this point demands the action of the debranching enzyme and is therefore slower, which partly explains the fact that the muscle can only perform its maximum exertion during a few.
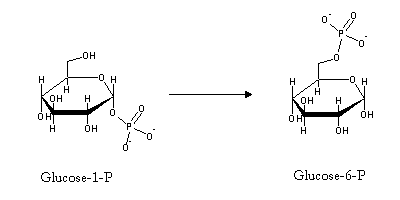
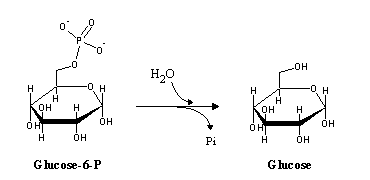
Glucose 6-phosphate can then be used in glycolysis. Unlike muscle, liver (and to a smaller degree, kidney) contains glucose-6-phosphatase, a hydrolytic enzyme catalyzing glucose-6-phosphate dephosphorylaton that allows it to supply glucose to other tissues:
 |
Biochemistry,
by Donald Voet & Judith Voet An excellent text. It presents Biochemistry with frequent references to organic chemistry and biochemical logic. Highly reccommended for students of Biochemistry, Chemistry and Pharmaceutical Sciences. |
 |
Biochemistry,
Stryer A widely used classical text, frequently updated and re-issued. |
 |
Textbook
of Biochemistry with Clinical Correlations, Thomas Devlin Strongly advised to students in Nursing, Medicine, Dentistry, etc. Plenty of examples of application of biochemical knowledge to clinical cases. |
 |
Principles
of Biochemistry, Lehninger A widely used classical text, frequently updated and re-issued. |