Associate Professor, Universidade Fernando Pessoa
| Other metabolic pathways: |
Besides being the most important building blocks of proteins aminoacids can also be used as precursors of nitrogen-containing molecules: hemes, nucleotides, glutathione, physiologically-active amines, etc.
Excess diet aminoacids are neither stored nor excreted as such: they are converted in pyruvate, oxaloacetate, a-ketoglutarate, etc. Therefore, aminoacids are also precursors of glucose, fatty acids, and ketone bodies, and can be used for energy production.
Aminoacid conversion involves the removal of the amine group (eamination), the incorporation of the ammonia produced in this reaction into urea for excretion and the conversion of the carbon skeletons into metabolic intermediates.
Deamination of most aminoacids involves a transamination step, i.e. the transfer of their amino groups to a a-ketoacid, thereby producing the aminoacid equivalent of the original a-ketoacid and the a-ketoacid equivalent of the original aminoacid. The amine acceptor is usually a-ketoglutarate, which is converted to glutamate:
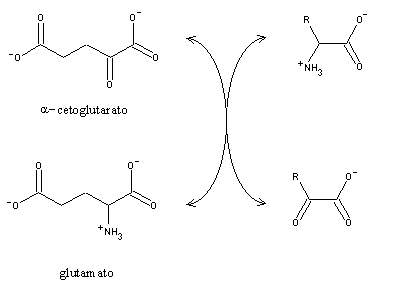
Aminotransferases use pyridoxal-5'-phosphate, a derivative of vitamin B6. Pyridoxal is also involved in reactions of aminoacid decarboxilation and removal of their sidechains. It is also the cofactor used by glycogen phosphorylase, although in this case the reaction mechanism is different. Aminotransferases are specific for each type of aminoacid, and produce the corresponding a-ketoacids. However, most accept only a-ketoglutarate or (to a minor extent) oxaloacetate as amine acceptor, yielding glutamate or aspartate, respectively. Therefore, most amine groups will eventually end in glutamate or aspartate, which can be interconverted by glutamate-aspartate aminotransferase.
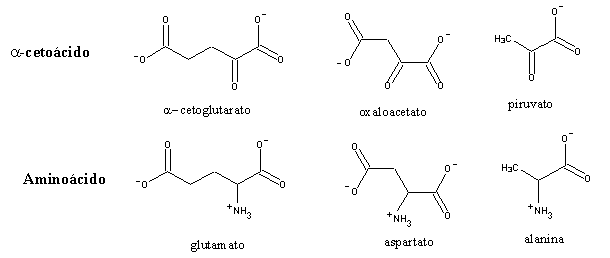
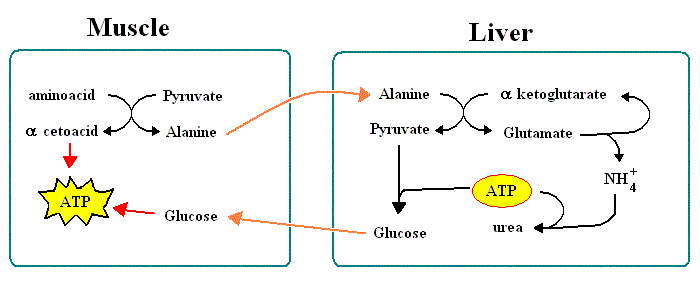
A set of muscle aminotransferases use pyruvate (which is also a a-ketoacid) as amine acceptor, producing the corresponding aminoacid, alanine . Upon release to the bloodstream, alanine is taken up by the liver, which transaminates it back to pyruvate, to be used in gluconeogenesis. The glucose produced in this process will be oxidized to pyruvate (and eventually lactate or CO2, depending on the conditions) by the muscle, thereby completing the alanine cycle. The released amino group will be used in urea synthesis. The net result of the alanine cycle is the transport of nitrogen from muscle to liver.
Transamination does not yield nitrogen release from aminoacids: it simply transfers the amino groups from a large variety of aminoacids into two or three aminoacids (glutamate, aspartate and alanine). Deamination is performed for the most part by glutamate dehydrogenase, a mitochodrial enzyme unique for its ability to use either NAD+ or NADP+.
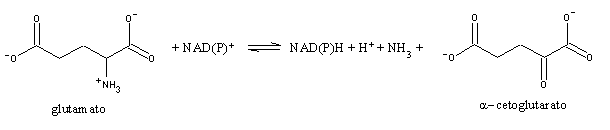
Nitrogen released by this reaction as ammonia must be excreted. Many water-living animals excrete it without modification. Other animals with less plentiful water supplies convert ammonia into less toxic products that need less water to be excreted. One of these products is urea.
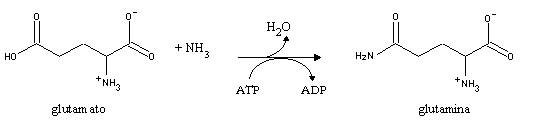
In order to replenish glutamate stocks, other aminoacids react with a-ketoglutarate by transamination. As a result of both reactions, a-ketoglutarate e glutamate progressively become exhausted, with very harmful consequences for neuron function (since glutamate is a precursor of neurotransmitters, and citric acid cycle operation is dependent on a steady level of intermediates like a-ketoglutarate).
Urea is synthesized in the liver, which secretes it to the bloodstream, whence it will excreted by the kidney. The global reaction of the urea cycle is:
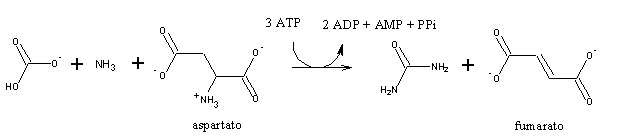
The first step is the synthesis of carbamoyl-phosphate, an activated form of nitrogen:
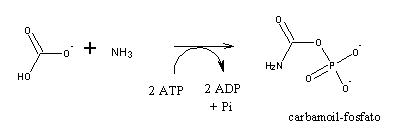
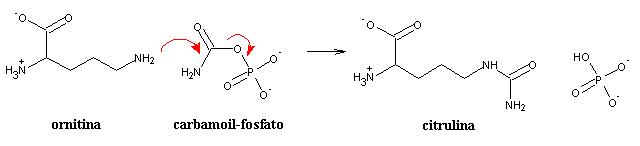
Carbamoyl is afterwards tranferred to ornithine, yielding citrulline. Bithe these molecules are "special" aminoacids, i.e., aminoacids which are not in the restricted set of 21 aminoacids used in protein synthesis.
After these two reactions (which occur inside the mitochondrion), citrulline is transferred to the cytoplasm, where the remainder of the cycle happens.
The second nitrogen atom in urea comes from aspartate:
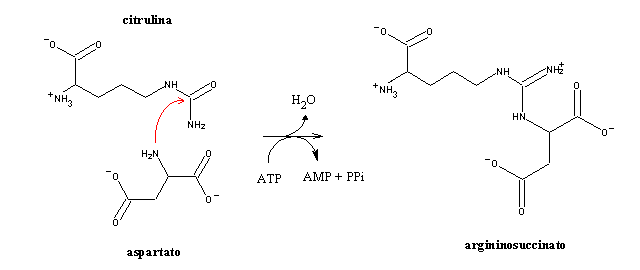
In this reaction, ATP is hydrolized to AMP, instead of ADP (as usually happens). Since AMP can accept a phosphate group from ATP, yielding 2 ADP, hydrolisis of ATP to AMP is equivalent to the hydrolysis of 2 ATP to 2 ADP.
Argininosuccinate can be cleaved into arginine and fumarate:
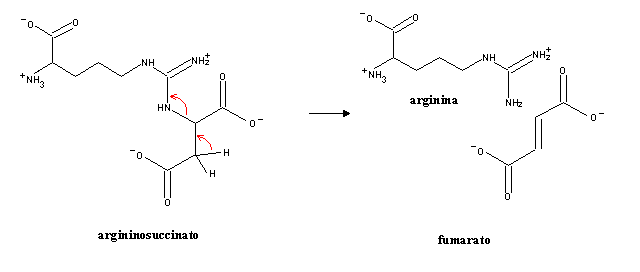
Upon entry into the mitochondrion, fumarate can react in the citric acid cycle to produce NADH and oxaloacetate, which can be converted into aspartate by transamination.
Arginine hydrolysis yields urea and ornithine, which can restart the cycle after entering the mitochondrion.
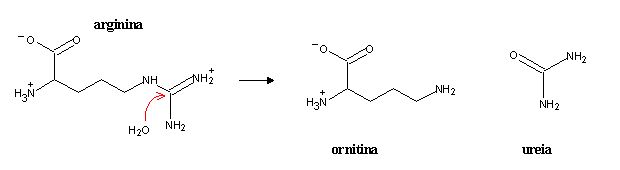
The urea cycle has a high energy cost, equivalent to the hydrolysis of 4 ATP to 4 ADP. However, this cost can be regained in the electron-transport chain, since the NADH produced in glutamate deamination and in the oxidation of fumarate to oxaloacetate are equivalent to about 6 ATP.
 |
Biochemistry,
by Donald Voet & Judith Voet An excellent text. It presents Biochemistry with frequent references to organic chemistry and biochemical logic. Highly reccommended for students of Biochemistry, Chemistry and Pharmaceutical Sciences. |
 |
Biochemistry,
Stryer A widely used classical text, frequently updated and re-issued. |
 |
Textbook
of Biochemistry with Clinical Correlations, Thomas Devlin Strongly advised to students in Nursing, Medicine, Dentistry, etc. Plenty of examples of application of biochemical knowledge to clinical cases. |
 |
Principles
of Biochemistry, Lehninger A widely used classical text, frequently updated and re-issued. |