REFERENCES:Previous Next Home
The role of hydrogen in the metabolism of Pyrococcus furiosus
P. furiosus is a strictly anaerobic Archaeon that grows near 100ºC by fermentation of carbohydrates through a modified Embden-Meyerhoff pathway, producing acetate, alanine, CO2 and H2 . Two soluble H2-producing enzymes are known in this organism: sulfhydrogenase I and sulfhydrogenase II . During metabolism of carbohydrates and amino acids, reducing equivalents are transferred to an iron-sulfur protein, ferredoxin. The oxidized form of ferredoxin must be regenerated in order to sustain the continuing operation of these metabolic pathways. However, the sulfhydrogenases from P. furiosus cannot use reduced ferredoxin as an electron donor, which prevents them from directly disposing of the reducing equivalents produced by the metabolism of P. furiosus. Instead, they are able to oxidize NADPH to NADP+ with concomitant production of H2. It was therefore suggested that these reducing equivalents may be transferred to NADP+ (thus generating NADPH) by a sulfide dehydrogenase, which can also function as a ferredoxin:NADP+ oxidoreductase . The produced NADPH will be used to reduce hydrogenase [1]. It has been shown that the reduction, catalyzed by enzymes, of protons to H2 by pyridine nucleotides is possible only when the partial pressure of H2 is kept below 0.1 kPa. P. furiosus is however able to grow under H2 accumulating conditions (pH2 > 30 kPa).
In order to understand the pathway of electron disposal in P. furiosus each of the enzymes involved (sulfide dehydrogenase and sulfhydrogenase) was purified and characterized. The physiological activities of these proteins in cell extracts were also investigated. The results of this study are succintly described below, and available in my PhD thesis.
SULFHYDROGENASE
Sulfhydrogenase revealed a very rich and intricate temperature-dependent redox chemistry. Although the enzyme has measurable H2-uptake activity at 45 ºC, analysis of the temperature-dependence of this activity suggests that the kinetic properties of the enzyme change markedly at 64 ºC [2] .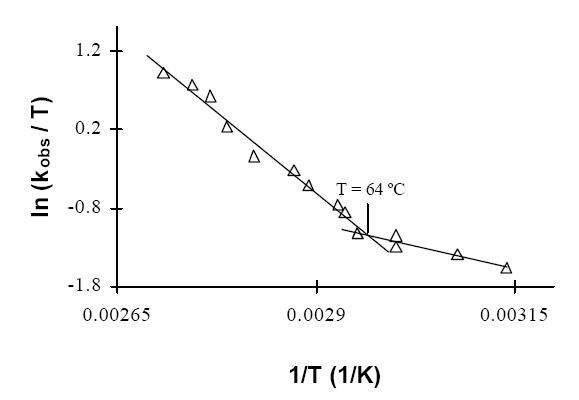
This may reflect a change of conformation of the protein or of the active site, e. g., the transition of the [NiFe] center from the "unready" to the "ready" state or a change of the redox potentials of the metal centers involved. Upon incubation of the oxidized sulfhydrogenase in the presence of H2 a remarkable increase in activity occurs. After activation the H2-evolution / H2-uptake ratio was observed to be less than unity [2], suggesting that the role of sulfhydrogenase in H2 evolution will not be as important as assumed in the model of Ma et al.[1] .
Incubation of anaerobically-oxidized sulfhydrogenase at 80 ºC revealed a complex process of temperature-induced self-reduction [3] . The identity of the internal reductant responsible for this process is not clear. Dithiothreitol seems to inhibit the self-reduction, suggesting the involvement of disulfide bridges in the process. This process has provided a very convenient way to generate different states of the [NiFe] active center for spectroscopic study.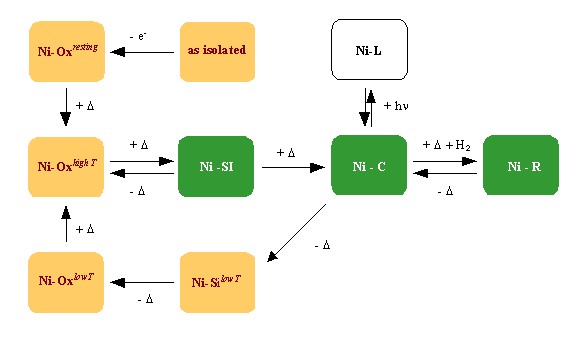
The temperature-induced activation (in orange) and catalytic cycle (in green) of sulfhydrogenase I. +D: anaerobic incubation at 80 ºC -D: anaerobic incubation at room temperatureMost of these states are heterogeneous, showing signals attributed to "ready" and "unready" hydrogenase. Their EPR spectra are also significantly more rhombic than the ones observed in mesophilic hydrogenases (cf. [4] and references therein). Several other interesting differences were also found. The Ni-C signal of P. furiosus sulfhydrogenase saturates much easier than the corresponding signals from Desulfovibrio hydrogenases and, though the Ni-C signal disappears upon illumination at low temperature as observed in regular hydrogenases, no new paramagnetic species (corresponding to Ni-L) were detected.
All of these data point to the existence of subtle differences in the electronic environment of the [NiFe] center of the P. furiosus enzyme. It is possible that some of these differences reflect an adaptation of the active site to the thermodynamic conditions under which it must operate.
The role of the detected [Fe-S] clusters is still not clear. Sequence analysis has indicated that probably more [Fe-S] clusters are present in the enzyme than have so far been examined by EPR. Whether these clusters are not detected due to very low redox potentials or to other unusual properties remains to be determined. The results of a redox titration at high temperature (PhD thesis, section 4.6) suggest that under some conditions one of the clusters cannot be reduced by dithionite, even though the solution potential is much lower than the redox potential of this cluster. The undetected clusters may have a similar behavior, e. g. kinetically hampered reduction due to inaccessibility from the solvent.
[1] Hydrogen production from pyruvate by enzymes purified from the hyperthermophilic archaeon, Pyrococcus furiosus: A key role for NADPH.K. Ma, Z.H. Zhou and M.W.W. Adams. FEMS Microbiol. Lett. (1994) 122: 245-250.
[2] Effects of temperature on the electron transfer between Pyrococcus furiosus hydrogenase and its redox partners. P.J. Silva, M.J. Amorim, P.-L. Hagedoorn, H. Wassink, H. Haaker and W.R. Hagen. Journal of Inorganic Biochemistry (1999) 74, 297.
[3] On the prosthetic groups of the NiFe sulfhydrogenase from Pyrococcus furiosus: topology, structure, and temperature-dependent redox chemistry. Pedro J. Silva, Baltazar de Castro and Wilfred R. Hagen. Journal of Biological Inorganic Chemistry (1999) 4, 284-291.
[4] Nickel hydrogenases: in search of
the active site. Simon P. Albracht. Biochim Biophys Acta
(1994) 1188, 167-204.