Assistant Professor, Universidade Fernando Pessoa
Metabolism is the set of chemical rections that occur in a cell, which enable it to keep living, growing and dividing. Metabolic processes are usually classified as:
There is a very large number of metabolic pathways. In humans, the most important metabolic pathways are:
Click on the picture to get information on each pathway
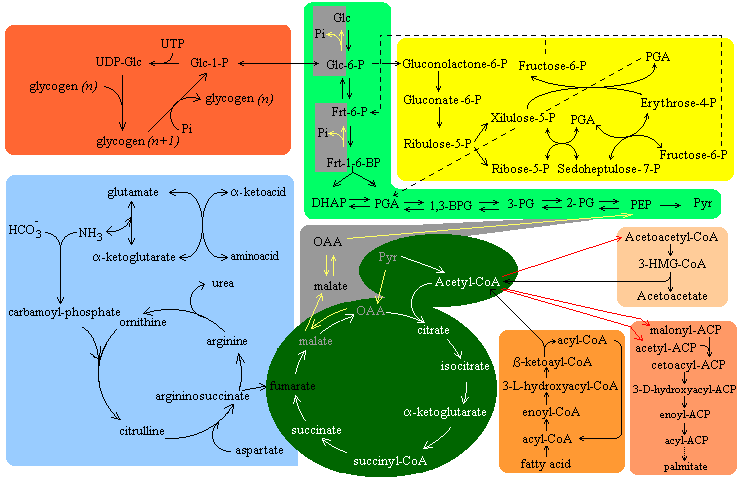
Metabolic pathways interact in a complex way in order to allow an adequate regulation. This interaction includes the enzymatic control of each pathway, each organ's metabolic profile and hormone control.
Metabolic flow through glycolysis can be regulated at three key points:
Flow is regulated in the gluconeogenesis-specific reactions. Pyruvate carboxilase is activated by acetyl-CoA, which signals the abundance of citric acid cycle intermediates, i.e., a decreased need of glucose.
The citric acid cycle is regulated mostly by substrate availability, product inhibition and by some cycle intermediates.
Carbamoyl-phosphate sinthetase is stimulated by N-acetylglutamine, which signals the presence of high amounts of nitrogen in the body.
Liver contains a hexokinase (hexokinase D or glucokinase)with low affinity for glucose which (unlike "regular" hexokinase) is not subject to product inhibition. Therefore, glucose is only phosphrylated in the liver when it is present in very high concentrations (i.e. after a meal). In this way, the liver will not compete with other tissues for glucose when this sugar is scarce, but will accumulate high levels of glucose for glycogen synthesis right after a meal.
Acyl-CoA movement into the mitochondrion is a crucial factor in regulation. Malonyl-CoA (which is present in the cytoplasm in high amounts when metabolic fuels are abundant) inhibits carnitine acyltransferase, thereby preventing acyl-CoA from entering the mitochondrion. Furthermore, 3-hydroxyacyl-CoA dehydrogenase is inhibited by NADH and thiolase is inhibited by acetyl-CoA, so that fatty acids wil not be oxidized when there are plenty of energy-yielding substrates in the cell.
Metabolic flow through the pentose phosphate pathway is controled by the activity of glucose-6-phosphate dehydrogenase, which is controlled by NADP+ availability.
Usually neurons use only glucose as energy source. Since the brain stores only a very small amount of glycogen, it needs a steady supply of glucose. During long fasts, it becomes able to oxidize ketone bodies.
The maintenance of a fairly steady concentration of glucose in the blood is one of the liver's main functions. This is accomplished through gluconeogenesis and glycogen synthesis and degradation. It synthesizes ketone bodies when acetyl-CoA is plenty. It is also the site of urea synthesis.
It synthesizes fatty acids and stores them as triacylglycerols. Glucagon activates a hormone-sensitive lipase, which hydrolizes triacylglycerols yielding glycerol and fatty acids. These are then released into the bloodstream in lipoproteins.
Muscles use glucose, fatty acids, ketone bodies and aminoacids as energy source. It also contains a reserve of creatine-phosphate, a compound with a high phosphate-transfer potential that is able to phosphorilate ADP to ATP, thereby producing energy without using glucose. The amount of creatine in the muscle is enough to sustain about 3-4 s of exertion. After this period, the muscle uses glycolysis, first anaerobically (since it is much faster than the citric acid cycle), and later (when the increased acidity slows phosphofrutokinase enough for the citric acid cycle to become non-rate-limiting) in aerobic conditions.
It can perform gluconeogenesis and release glucose into the bloodstream. It is also responsible for the excretion of urea, electrolytes, etc. Metabolic acidosis may be increased by the action of the urea cycle, since urea synthesis (which takes place in the liver) uses HCO3-, thereby further lowering blood pH. Under these circunstances, nitrogen may be eliminated by the joint action of kidney and liver: excess nitrogen is first incorporated in glutamine by glutamine synthetase. Kidney glutaminase then cleaves glutamine in glutamate e NH3, which the kidney immediately excretes. This process allows nitrogen excretion without affecting blood bicarbonate levels.
 |
Biochemistry,
by Donald Voet & Judith Voet An excellent text. It presents Biochemistry with frequent references to organic chemistry and biochemical logic. Highly reccommended for students of Biochemistry, Chemistry and Pharmaceutical Sciences. |
 |
Biochemistry,
Stryer A widely used classical text, frequently updated and re-issued. |
 |
Textbook
of Biochemistry with Clinical Correlations, Thomas Devlin Strongly advised to students in Nursing, Medicine, Dentistry, etc. Plenty of examples of application of biochemical knowledge to clinical cases. |
 |
Principles
of Biochemistry, Lehninger A widely used classical text, frequently updated and re-issued. |