

Kainite
A whitish monoclinic mineral: MgSO4KCl3H2O or KMg (SO4)Cl3H20. It is a natural salt occurring in irregular granular masses, and is used as source of potassium and magnesium compounds.

Is chemical a formula is KMg[SO4]Cl,3 H2O or MgSO4,KCl,3 H2O. It crystallizes in the form of prisms in the monoclinic system. It is, usually, in the form of compact granular masses. The crystals are rare and, when they exist, in tabular or prismatic facies. Its colour is white, yellowish or greyish, sometimes red. Its shine is glassy. The hardness is 2 and the density of 2.1 g / cm 3 . It melts with a torch and is easily soluble in water. Its taste is bitter and salty. It is non-hygroscopic, which differentiates it from carnalite. It is widespread in the Stassfurt salt deposits in association with schoenite K2Mg [SO4]2 6 H2O, carnalite, kieserite, halite. It constitutes a raw material for the preparation of fertilizers and the production of potassium salts. (https://www.universalis.fr/encyclopedie/kainite/#i_0).
Kieserite
A white monoclinic mineral: MgSO4H20. It occurs in saline residues.

Kieserite is a natural mineral that is chemically known as magnesium sulfate monohydrate (MgSO4 H2O). It is extracted from geological marine deposits and provides a soluble source of both Mg and S for plant nutrition. Kieserite is mainly obtained from deep underground deposits of minerals in Germany. She is here in the remnants of ancient oceans that have evaporated and are now buried beneath the Earth's surface. These resources ancient oceans that were evaporated and are now buried beneath the Earth’s surface. These mineral resources contain a variety of valuable plant nutrients. The ore is brought to the surface where magnesium salts are separated from potassium and sodium salts using a unique, electrostatic process. (http://www.ipni.net/publication/nss-fr.nsf/0/40640A7D75987DDB852582110074E593/$FILE/NSS-FR-8.pdf).
Kinematic Ratio R°/A°
Kinematic or n molding ratio (R°/A°) is the, flow rate of salt minus dissolution (R°) divided by aggradation rate minus compaction (A°).
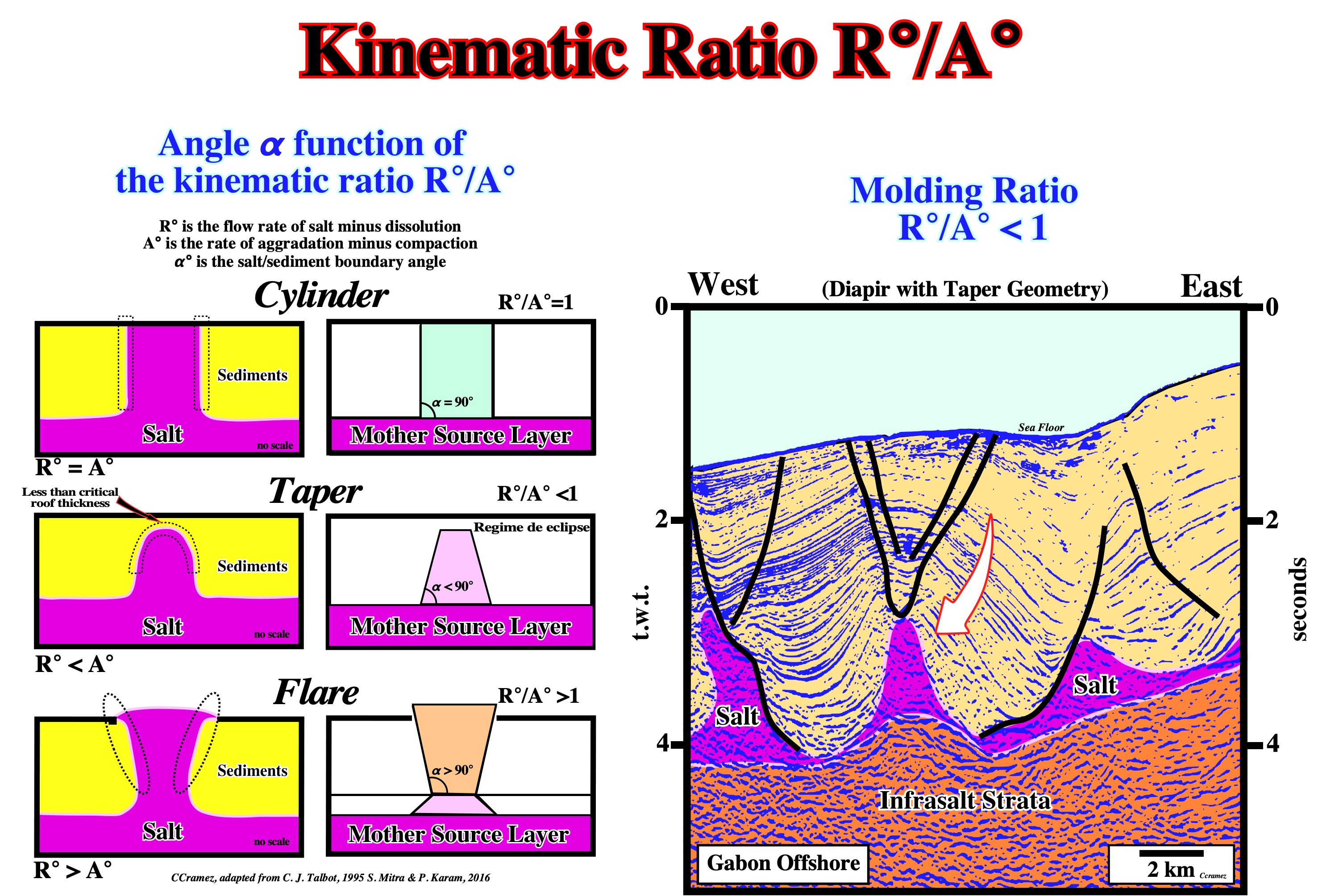
Theoretical three salt/sediment boundary angles are recognized function of the molding ratio. (i) Cylinder (R°/A° = 1), structures with flanks more or less vertical ; (ii) Taper ((R°/A° < 1) when the flanks are, roughly, convergent upward and (iii) Flare ((R°/A° > 1), when the flanks of the salt structures are divergent upward. Thus, on the tentative geological interpretation of a Canvas autotrace of Gabon offshore seismic line, the central salt diapir with a taper geomety suggests a local kinematic ratio (R°/A°) lower than 1.
Kongruent Fold Style
Tectonic fold style recognized in the overburden of the Permian Zechstein salt characterized by broad antiforms and synforms.
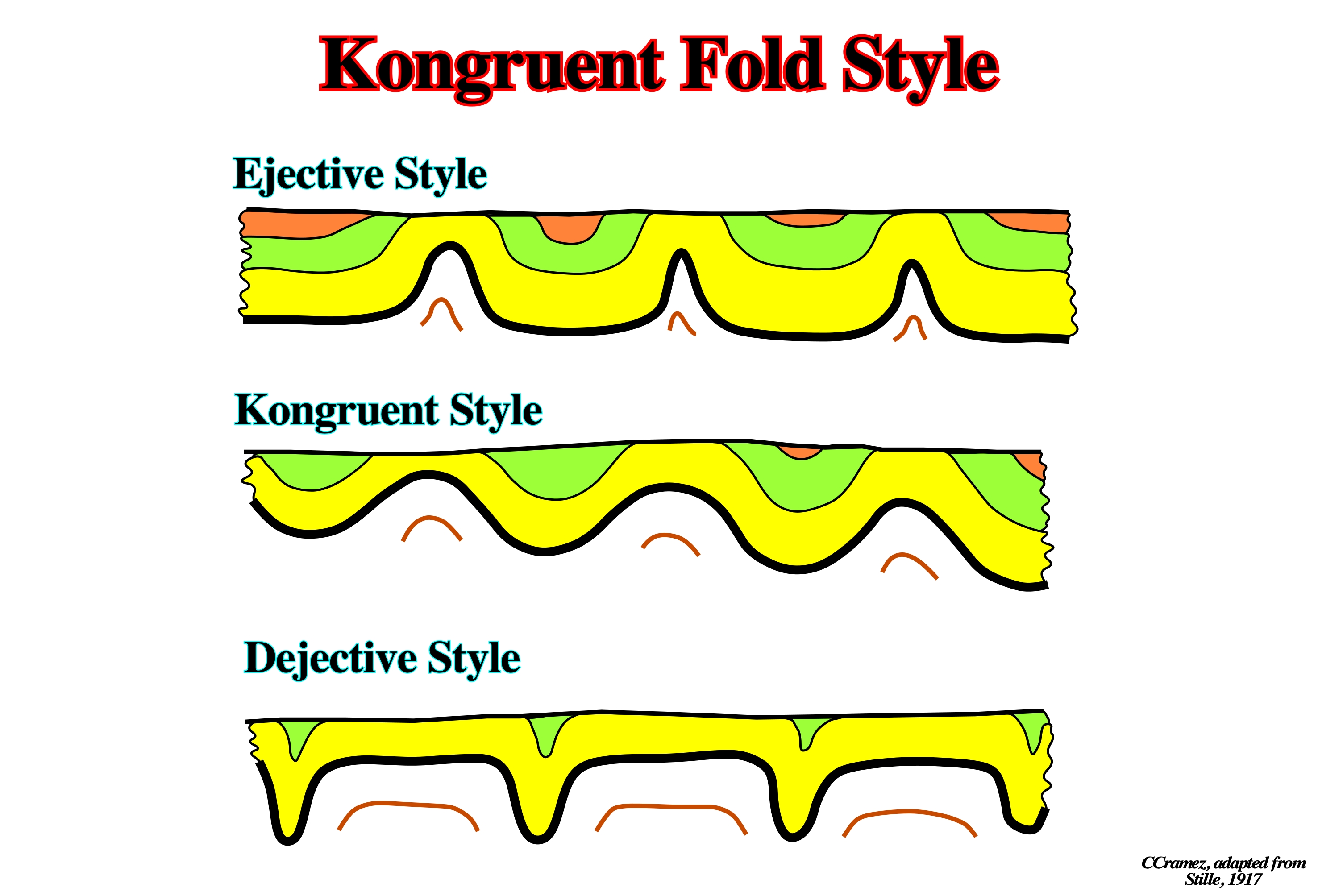
Stille (1917), identified three major geometries of folds decoupling over Permian Zechstein: (i) Ejective, characterized by narrow antiforms and broad, flat-bottomed synforms, (ii) Kongruente, characterized by an intermediate geometry between the two end-members, and (iii) Dejective, characterized by narrow synforms and broad, flat-topped antiforms.